Introduction
Multiple sclerosis (MS) is a chronic autoimmune disease of the central nervous system that progressively affects the brain and spinal cord [1, 2]. MS is characterised by degeneration of the myelin sheath, leading to plaque formation and disruption of action potential flow [3]. Approximately 2.8 million people are estimated to live with MS worldwide, with a prevalence rate of 35.9 per 100,000 population [4]. Specifically regarding the population studies, in 2020 the number of Iranian MS patients was estimated to be 75,000, with a prevalence rate of 90 per 100,000 [5]. In 2020, the prevalence in Tehran was 167.54 cases per 100,000 people, with higher rates among women (252.65 per 100,000) compared to men (83.15 per 100,000) [6].
The development and progression of MS involve various factors that play a significant role in disease initiation and progression, including immune mechanisms [7]. Cytokines produced by the immune system are particularly important and may serve as primary targets for therapeutic interventions in MS [8].
Cytokines can be divided into four subgroups that might interact with MS in different ways: Th1 inflammatory cytokines (likely responsible for initiating central nervous system inflammation), Th2 anti-inflammatory cytokines (associated with disease progression control), Th17 inflammatory cytokines (contributing to inflammation and tissue damage), and Treg cytokines (regulating the immune system) [9]. Interleukin-17 (IL-17), secreted by Th17 cells, plays a central role in brain inflammation, interfering with myelin regeneration and survival [10, 11], and indirectly influencing the production of cytokines directly correlated with MS progression, such as IL-6, IL-8, and IL-4 [11]. Interleukin-10 (IL-10), a regulatory cytokine produced by Th2 cells, suppresses inflammation and acts as an inhibitor of T cell activity, influencing cell-dependent immune responses [12, 13]. Considering that IL-10 levels decrease before disease relapse and gradually increase during the recovery phase [14], it might serve as a biomarker for disease stage and treatment response [15]. Calcium-binding protein (S100) may also be a relevant marker, as it is associated with blood-brain barrier permeability [9], which has been associated with MS pathogenesis [16].
Due to the involvement of the motor cortex and spinal cord, MS is associated with important limitations in physical function, such as muscle weakness, imbalance, fatigue, and spasticity, which significantly affect overall performance and quality of life [1, 17]. In this regard, non-pharmacological methods have gained attention [18, 19], especially exercise training, as it has been shown to promote improvements in the physical function and quality of life of patients with MS [17, 20]. Exercise may also modulate anti-inflammatory and proinflammatory cytokines, which may be valuable in managing MS [9]. However, the effects of exercise on immune and inflammatory markers in MS are not yet fully understood, since some studies indicated a positive impact of exercise training on cytokine regulation, release of neurotrophic factors, and fatigue in MS patients, while others have reported no significant changes [9, 21].
Water-based exercises might be particularly useful for patients with MS, as they provide a safe environment that reduces the fear of falling, offers joint support, diminishes gravitational force, and helps postural control [22, 23]. Another important aspect of water-based exercise is temperature regulation, as temperature increases are related to worsening symptoms in patients with MS [2, 24]. Previous studies have shown that aquatic exercise might improve the quality of life, balance, gait, and fatigue of patients with MS [23, 25, 26]. However, we are not aware of any studies analysing the effects of different water-based exercise protocols on cytokine levels in patients with MS, and investigating training intensity may be particularly valuable. Although low-to-moderate-intensity exercises are commonly recommended for patients with MS [2], other studies have suggested that individuals with MS can tolerate and adhere to high-intensity training, resulting in significant functional improvements [27, 28], even better than those obtained by low-intensity protocols [29]. In contrast, Hortobágyi et al. [30] suggested that high-intensity exercise is superior to low-intensity exercise in improving neuroplasticity and motor outcomes in healthy young adults but not in older adults or clinical populations. Therefore, it is important to compare the different exercise protocols to provide evidence-based recommendations.
The objective of this study was to compare the effects of eight weeks of water-based exercise training with lower and higher intensities on the IL10, IL17, and S100 indices, disability scores, and functional performance in women with MS. Our hypothesis is that both groups will improve their functional capacity and inflammatory markers compared to the CON group, with no difference between them.
Material and methods
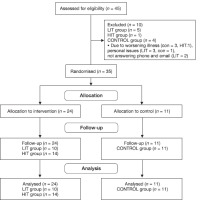
This study employed a semi-experimental design with pre- and post-test approaches, including a CON group. Participants were recruited from women with MS who sought treatment at neurology clinics affiliated with the Isfahan University of Medical Sciences and the Isfahan MS Association. From this population, a purposive sampling method was used to select 45 women with relapsing-remitting MS and an Expanded Disability Status Scale (EDSS) score < 3.5. the sample size was determined using G Power software, with an effect size of 0.25, statistical power of 0.8, and correlation among variables of 0.5, for a 2 x 3 within-between ANOVA, resulting in 30 participants. We opted to recruit 45 participants to account for attrition and dropout.
The inclusion criteria were as follows: (1) a minimum of 2 years of MS diagnosis confirmed by neurological examination and magnetic resonance imaging (Mr) performed by a neurologist; (2) an EDSS score < 3.5; (3) females aged between 30 and 45 years; (4) no disease recurrence in the 4 weeks preceding the study and during the training period; (5) no comorbidities that could interact with the study protocol, such as heart, vascular, respiratory, skin, joint, or neuromuscular diseases; (6) ability to consistently attend training sessions; (7) non-participation in other rehabilitation and sports programs; and (8) regular menstrual cycles. Exclusion criteria included (1) missing more than five exercise training sessions, (2) disease recurrence during the study, (3) diseases or conditions that could be aggravated by the study protocol, (4) non-participation in examination sessions, and (5) irregular menstrual cycles.
The participants were randomly divided into three groups of 15 participants each: low-intensity water-based aerobic training (50-65% of the maximum reserve heart rate, LIT), high-intensity water-based aerobic training (70-85% of the maximum reserve heart rate, HIT), and a CON group (CON, 15 participants).
Their characteristics are shown in Table 1. The exercise groups completed water-based exercise sessions three times a week, lasting 30-60 minutes per session, for a total of eight weeks (Table 2). The CON group was instructed to maintain a normal daily routine throughout the study. Of the initial 45 volunteers, five from the LIT exercise training group, one from the HIT group, and four from the CON group were excluded because of disease recurrence, family problems, and irregular participation in the exercise training program. The statistical analysis involved data from 35 individuals (10 in the LIT group, 14 in the HIT group, and 11 in the CON group, Figure 1).
Table 1
Demographic and clinical characteristics of the participants
Table 2
Water training protocol
Volunteers filled out personal and medical forms and received a detailed explanation of the research procedures. In cases of agreement, participants provided written consent, confirming their voluntary participation in the study.
Training program
The exercises used in this study are presented in Table 2. The intensity was based on the estimated maximum heart rate reserve (HRR) using the following formula (HRR = maximum heart rate - resting heart rate) [31]. the intensity progressed for Lit and Hit groups as follows: weeks 1 and 2 (50 and 70% HRR), 3 and 4 (55 and 75% HRR), 5 and 6 (60 and 80% HRR), 7 (65 and 85% HRR), and 8 (69 and 95% HRR). Each set of exercises lasted for one minute. Five sets were performed in weeks one to two, and the number of sets increased every two weeks until it reached eight by weeks seven and eight (Table 2). The rest between sets ranged from 30 to 45 seconds, depending on each patient’s ability. Total training duration was between ~45 to 75 minutes, from the first to last week.
Functional assessments
Disability was assessed by a neurologist using the EDSS questionnaire, which assigns a score between 0-10 based on different parameters [32].
Functional performance was measured using the standard tests proposed by Rickley and Jones [33], as follows:
Lower body muscle strength (30-second sit-to-stand test): total repetitions performed in 30 seconds of sitting and standing up from a 42 cm chair, with arms crossed over the chest.
Upper body muscle strength (elbow flexions): total number of elbow flexions performed with 2.5 kg dumbbells in a sitting position.
Cardio-respiratory endurance (2-minute walking distance): walking as fast as possible for two minutes out and back on a 30 m track, distance was measured with a measuring wheel.
Dynamic balance (timed up and go): participants started sitting on a 42 cm chair and, at the command, got up from the chair without using their hands, walked for eight feet (2.44 m), and returned to the chair. Time was measured with a stopping watch.
All tests were conducted by the same researcher, who was blinded to group allocation, and between 8 and 10 AM. Cronbach’s alpha was used to determine the internal reliability of these tests, and the values ranged from r = 0.78-0.84.
Blood analysis
Blood samples were collected 24 hours before the first training session and 48 hours after the final training session at 8:00 AM while in a fasted state. The collected samples were poured into tubes containing blood anticoagulant (EDTA), then immediately centrifuged at 2000-3000 rpm at 37°C for 10 minutes, and the plasma was separated from it. Baseline samples were stored in a freezer at -80°C and analysed when posttest measurements were obtained. The plasma protein concentrations of S100, IL-10, and IL-17 were measured using ELISA kits (ZellBio GmbH, ZellBio Company, Germany). The kits had a sensitivity of 5 ng/ml for S100, and the intra- and inter-assay variation coefficients were less than 12% and 10%, respectively. The kits for IL-10 and IL-17 had sensitivities of 2.5 ng/mL and 1 ng/mL, respectively, with intra-assay and interassay variation coefficients less than 12% and 10%.
Statistical methods
The normality of the data was confirmed using the Shapiro-Wilk test (p > 0.05). Data are presented as mean and standard deviation. The homogeneity of variances was examined using Levene’s test. The significance level ofp > 0.05 from Levene’s test suggested that the data exhibited homogeneity, thus enabling the use of a parametric statistical method. Within-group comparisons were performed using paired f-tests. Between-group comparisons were tested using ANCOVA by comparing post-intervention values, using baseline values as covariates. Bonferroni tests were used as post hoc tests when necessary. Statistical analysis was conducted using the SPSS version 21 software, and the significance level was set at p < 0.05.
Results
The results for each group are shown in Table 3 as means and standard deviations.
Table 3
Descriptive statistics in the studied groups
EDSS
EDSS scores significantly decreased by 41.3% and 53.3% for the HIT and LIT (p < 0.01) groups, respectively, and increased by 40.5% for the CON group (p < 0.05). the EDSS changes significantly differed between the groups (p < 0.01). the changes were higher in the LIT and HIT groups than in the CON group (p < 0.01). there was no significant difference between the Lit and Hit groups (p > 0.05).
Muscle strength
The number of elbow flexions performed in 30 seconds with a 2.5 kg dumbbell increased by 44.3% and 36.8% for the HIT and LIT groups (p < 0.01), respectively, and did not change for the CON group (-10.1%; p > 0.05). Changes were significantly different between the groups (p < 0.01), being higher for the LIT and HIT groups than the CON group (p < 0.05 and p < 0.01, respectively). There was no significant difference between the LIT and HIT groups (p > 0.05).
The number of repetitions performed in the 30-second sit-to-stand test increased by 20.5% and 30.3% for the HIT and LIT groups (p < 0.01) and did not change for the CON group (-6%; p > 0.05). Changes were significantly different between groups (p < 0.01), being higher for the LIT and HIT groups compared to the CON group (p < 0.05 and p < 0.01, respectively). There was no significant difference between the LIT and HIT groups (p > 0.05).
Cardiorespiratory endurance
The maximum distance covered in 2 minutes increased by 23.7% and 62.9% for the HIT and LIT group (p < 0.01) and did not change for the CON group (-8.5%; p > 0.05). Changes were significantly different between the groups (p < 0.01). The changes were higher in the LIT and HIT groups than in the CON group (p < 0.01). There was no significant difference between the LIT and HIT groups (p > 0.05).
Dynamic balance
The time to complete the timed up-and-go test decreased by 31.1% and 52.7% for the HIT and LIT groups (p < 0.01) and increased for the CON group (71.6%, p < 0.05). There were significant differences between groups (p < 0.01). The changes were higher in the LIT and HIT groups than in the CON group (p < 0.01). There was no significant difference between the LIT and HIT groups (p > 0.05).
Inflammatory markers
IL-10 levels did not change for the HIT and LIT groups (1.9 and -4.3%, respectively; p > 0.05) and increased for the CON group (15.4%; p < 0.05). There was no difference between groups for changes in IL-10 levels (p > 0.05).
IL-17 levels did not change for the HIT and LIT groups (3.6 and -0.6%, respectively; p > 0.05) and increased for the CON group (9%; p < 0.05). There was no difference between groups for changes in IL-17 levels (p > 0.05).
The IL-10/IL-17 ratios did not change in any group (-0.4%, -2.2%, and 6.2% for the HIT, LIT, and CON groups, respectively; p > 0.05). There was no difference between groups for changes in IL-10/IL-17 ratios (p > 0.05).
S100B levels did not change in any group (-2.9%, 3.5%, and 6.7% for the HIT, LIT, and CON groups, respectively; p > 0.05). There was no difference between groups for changes in S100B levels (p > 0.05).
Discussion
This study aimed to compare the effects of two different intensities of aquatic exercise training on IL-10/IL-17 ratios, S100B indices, disability scale scores, and physical performances in women with MS. The results demonstrated a significant improvement in functional indices and EDSS in both training groups compared to the CON group after eight weeks, which agrees with previous studies [34, 35]. An increase in muscle function, even in the absence of high external loads, may occur because water resistance increases muscle function when moving the body through water [35]. This is in agreement with previous studies suggesting that exercise training with no external load and aerobic-based activities might promote increases in neuromuscular function when performed at high-intensity effort [36, 37]. Water immersion may be particularly interesting because it improves muscle blood flow and allows exercise at higher intensities and longer durations, especially in clinical populations [38]. Other factors associated with the aquatic environment include increased venous return, central venous pressure, and diastolic filling, which may favour cardiorespiratory and skeletal muscle adaptation [39].
When comparing different training intensities, our results showed no differences between the LIT and HIT groups. This is in agreement with Hortobágyi et al. [30], who suggested that although high-intensity exercise might be superior to low-intensity exercise for improving motor outcomes in healthy young adults, this does not seem to be the case in clinical populations. This might have important clinical applications, as Hit training might impact adherence to exercise in patients with MS.
The anti-inflammatory effect of exercise training in chronic diseases is thought to be mediated by a decrease in the concentration of proinflammatory cytokines such as IL-6, IL-8, TNF-a, and IL-17 and an increase in the concentration of the anti-inflammatory cytokine, IL-10 [9, 21]. Exercise training may have therapeutic potential in the treatment of neuroinflammatory and neurodegenerative diseases [9, 21], especially MS, an inflammatory CNS demyelinating disease characterised by an imbalance between proinflammatory and anti-inflammatory cytokines [40]. However, the effect of exercise on inflammatory markers remains unclear. regarding MS, changes in inflammation markers might be difficult to detect because MS is already an inflammatory condition that could mask the beneficial effects of physical activity.
However, the lack of changes in IL-17 and IL-10 concentrations in the exercise groups with simultaneous changes in the CON group may indicate a beneficial effect of the applied training, blocking further inflammation. Some anti-inflammatory mechanisms of physical exercise have been previously presented. Exercise mobilises regulatory T (T reg) cells, which are a major source of the anti-inflammatory cytokine IL-10, and decreases the ratio of inflammatory to anti-inflammatory cytokines [6]. Additionally, exercise training can reduce circulating levels of inflammasome activation-related inflammatory cytokines such as IL-β and IL-18, particularly in overweight/obese populations [41]. Taken together, these suggest that exercise training can be particularly beneficial in the setting of chronic inflammation and its related risks [42].
The lack of an effect on the S100B index and IL10/IL17 ratio is in agreement with Negaresh et al. [9, 21], who suggested that, in general, physical activity and exercise do not affect cytokine profiles in MS. This finding suggests that the mechanism by which exercise improves functional outcomes in MS is unrelated to the cytokines studied. regarding the effect of regular exercise training on IL-10 levels in patients with MS, there have been few studies, most of which reported no significant changes, similar to the results of the present study [21, 22, 43]. However, some studies have reported decreases [44] and increases [45] in IL-10 levels following exercise training. The controversial results regarding anti-inflammatory indices may be influenced by factors such as fat tissue, initial physical fitness, and duration of the training period [43]. Therefore, one possible reason for the lack of effectiveness of regular physical activity in IL-10 in these studies, including the present study, could be the short training period (often less than eight weeks) and lower fitness levels of individuals with MS compared to those of healthy individuals.
IL-17 is a key regulator of the relationship between adaptive and innate immunity and has been shown to play a role in MS by disrupting the blood-brain barrier [46, 47]. In contrast to the present study, studies in morphine-dependent rats [48] and healthy and asthmatic rats [49] reported a decrease in IL-17 levels after training. A possible reason for the discrepancy in the results could be the difference in the type of exercise training. Heydarianpour et al. suggested that the decrease in IL-17 levels after exercise training might be attributed to increased serum IFN levels and the inhibitory effect of cytokines on Th17 development.
Some important limitations of the study need to be highlighted. The study did not measure body composition, which would help to explain the results, considering that IL-17-producing cells, such as T cells, have been identified in adipose tissues [48]. Also, the lack of a CON group for the conditions (a group of training or not training individuals without MS) is a limitation that could help explain the results of inflammatory markers. This leads to the suppositions that either the treatment (exercise) does not affect these markers or the inflammatory markers are already at a different standard in patients with MS. Perhaps if the CON group was examined, the reason for the lack of change in inflammatory factors in the exercise groups would be explained, which was one of the limitations of the present study.
Conclusions
Low- or high-intensity water-based exercises resulted in similar functional benefits in women with MS. Based on this, one can choose between both types, which could have important practical applications because lower-intensity activities might result in fewer barriers and increase participation.