Introduction
Hormones are largely responsible for the integrated communication of various physiological systems that modulate cell growth and development. However, specific hormonal influences must be considered in the context of the entire endocrine system and its relationship with other physiological systems. Testosterone, growth hormone (GH), and insulin-like growth factor-I (IGF-1) are the three anabolic giants in cell growth and repair [1]. The serum concentrations of these hormones can directly affect athlete performance and reflect their training status during preparation [2].
IGF-1 and its binding protein insulin-like growth factor binding protein 3 (IGFBP-3) have been shown to be sensitive markers of overtraining and nutritional status [2]. Moreover, some studies have shown that intense exercise can lead to a significant decrease, rather than the expected increase, in circulating GH and IGF-1 concentrations, accompanied by an increase in cortisol and cytokines [3-6]. A possible explanation involves the elevation of cytokines induced by intense exercise sets. Cytokines, including interleukin-6 (IL-6), interleukin-1 β (IL-1P), interleukin-1ra (IL-1ra) and tumour necrosis factor-alpha (TNF-α), are known to directly inhibit the anabolic activity of the GH/IGF-1 axis [5, 7], while muscle glycogen content influences the magnitude of IL-6 release during exercise [8]. As glycogen stores approach total depletion, glycogenolysis and glucose transporters in muscle and liver are downregu- lated, along with IGF-1 production. As such, transient resistance to insulin develops during exercise under conditions of glycogen depletion [9].
In this scenario, carbohydrate supplementation may be an interesting alternative to avoid metabolic situations that may affect an athlete’s performance. For some time, the literature has pointed to the impact of carbohydrate supplementation on the performance of athletes in endurance races [10, 11]. Carbohydrate supplementation has been shown to enhance running performance, particularly in endurance events lasting over an hour. By maintaining higher blood glucose levels and preserving muscle glycogen stores, carbohydrate intake helps delay the onset of fatigue, allowing athletes to sustain higher intensities for longer periods. Additionally, it aids in faster recovery by replenishing glycogen stores post-exercise, promoting better performance in subsequent training sessions or races [12].
Although the existing literature has explored the relationship between GH and IGF-1 and carbohydrate supplementation, the acute effects of supplementation on the changes in serum concentrations of the GH/IGF-1 axis in runners have not yet been documented. Based on this, the study acutely examined the behaviour of GH, IGF-1, cortisol, insulin, and glucose serum concentrations and the race time of runners in a simulated road race with and without carbohydrate supplementation. We hypothesised that carbohydrate supplementation would improve runner’s performance (reducing total time), elevate serum glucose concentrations, decrease serum cortisol and insulin concentrations, and not affect serum GH and IGF-1.
Material and methods
Participants
The study involved 11 male runners (age = 40 ± 2 years; height = 1.76 ± 0.05 m; body mass = 71.4 ± 8.36 kg) with a mean training time of seven years and weekly training frequency of five to seven sessions. The sample was non-randomly selected and consisted of volunteer athletes who trained regularly. G*Power 3.1.9.7 calculated the sample size (minimum size, n = 10; effect size, d = 2.897) using pre- and post-intervention target serum glucose levels (70-100 mg/dl and 100125 mg/dl, respectively).
The inclusion and exclusion criteria were (a) active male recreational runner, (b) able to run 13 km, (c) practised running at a recreational level for at least the past two years, (d) perform endurance training regularly, with a weekly frequency of no less than three, (e) no chronic diseases or ongoing injuries, (f) and attended a medical check-up in the last six months.
Data collection
After being informed verbally and in writing, using an informed consent form, about the procedures adopted in the study, the athletes underwent an anthropometric assessment to determine their weight and height. Blood samples were taken before the start of each simulated race, after 30 min of rest, and 30 min after the end of the race, totalling two venipunctures.
The runners participated in two simulated road races over a distance of 13 km. In one simulated race (CARB), the runners were randomly assigned to consume a drink containing carbohydrates in the form of maltodextrin powder (120 g) diluted in 600 mL of water or a 600 mL placebo drink with the same colour and flavour as the maltodextrin-containing drink.
In the other simulated race (PLAC), which took place seven days later, the athletes who had used maltodex- trin in the first race consumed only a 600 mL placebo drink with the same colour and taste as the maltodex- trin-containing drink, and vice versa.
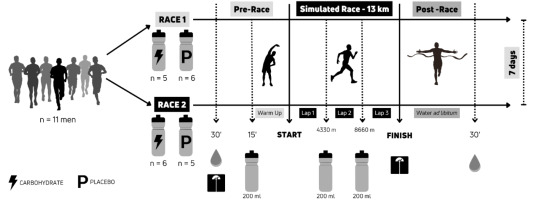
The athletes drank 200 mL of the maltodextrin- containing drink (40 g) or the placebo drink 15 min before the start of the race, 200 mL after completing 4330 m (first lap), and 200 mL after completing 8660 m (second lap). Before data collection, participants fasted for eight hours and ate no meals between blood collections. The experimental design is shown in Figure 1.
Thirteen-kilometre simulated road race
The course was a 4330 m running track (altitude 968 m, total positive and negative elevation of ~100 m), so there were three complete laps and an additional 10 m added at the end of the last lap. Two global positioning system (GPS) devices, a Garmin 820 edge cycle computer and a Garmin 735xt watch, measured distance. The ambient temperature was 11.7°C (95.0% relative humidity) for race one and 10.4°C (95.0% relative humidity) for race two.
Anthropometric measurements
A portable stadiometer (Sanny) and an electronic scale (TANITA BC-558) measured height and body mass, with all measurements performed by the same assessor.
Blood sample collection and analysis
Venous blood was collected from the forearm in tubes with and without an anticoagulant (ethylenedi- aminetetraacetic acid [EDTA]) (5 ml/tube) before and after testing (10 ml pre-test and 10 ml post-test). After centrifugation, the plasma and serum samples were stored at -80°C for later analysis. All analyses were performed at the BonneSantéLab Laboratory (Pratápo- lis, MG, Brazil). The intra-assay coefficients of variation were 2.77% for IGF-1, 2.80% for GH, 2.34% for cortisol, 2.93% for glucose, and 2.58% for insulin.
Serum GH and IGF-1 determination
Serum concentrations of GH and IGF-1 were determined by specific immunoassays using commercially available kits (Immulite 2000, Siemens, CA, USA). All study samples were dosed in duplicate in the same assay.
Serum cortisol and insulin determination
A commercial kit, Cortisol Coat-A-Count (DPCM- edlab®, Sao Paulo, Brazil), was used for cortisol determination. Insulin levels were measured by solid phase radioimmunoassay using the commercial Coat-A-Count kit (Siemens, CA, USA). All study samples were dosed in duplicate in the same assay.
Serum glucose concentration determination
Glucose analyses were performed using a Human kit on a Human HS200 device with Human’s own control and calibrator.
Statistical analysis
The data were presented as mean and standard deviation (SD), with the Shapiro-Wilk test verifying the normality of distribution. Student’s t-tests compared the intervention time points (pre and post) for parametric data, while Wilcoxon’s non-parametric test assessed non-parametric variables (insulin, IGF-1, and GH). For comparisons between interventions (placebo and carbohydrate), an independent Student’s t-test was used for cortisol concentrations, and the Mann-Whitney U-test was applied to the remaining variables. The effect size was calculated using the equations below.
Student’s t-test,
Mann-Whitney U and Wilcoxon, r = z/Vn.
The significance level was set at p < 0.05.
Results
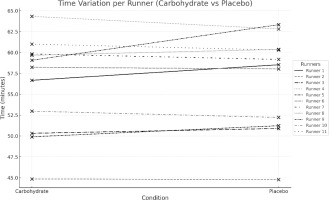
Figure 2 shows the times achieved by the runners in the two simulated 13 km races run seven days apart.
No difference was observed in the runners’ race time when comparing the results obtained for the two races. The Student’s t-test for paired samples showed that the mean race times (CARB: 56.07 ± 5.83 min; PLAC: 56.52 ± 5.87 min) were not significantly changed after carbohydrate supplementation (p = 0.384).
Table 1 shows the mean GH, IGF-1, cortisol, insulin, glucose, and body mass values before and after the two simulated road races, with and without carbohydrate supplementation (mean ± SD).
Table 1
Comparison of the mean GH, IGF-1, cortisol, insulin, glucose, and body mass values measured in two simulated running tests performed with and without carbohydrate supplementation
GH and cortisol levels increased significantly (pre- vs post-) in both the CARB and PLAC running situations (GH: p = 0.03; p = 0.001; cortisol: p = 0.001; p = 0.004, respectively). IGF-1 did not show statistically significant changes in any of the situations studied.
An increase in glucose levels was observed in the CARB run (p = 0.01). In turn, insulin concentrations increased in the CARB run (p = 0.05) and decreased in the PLAC run (p = 0.03).
A significant decrease in body mass was observed in both situations (p = 0.001 and p = 0.001).
Discussion
The study hypothesised that carbohydrate supplementation would improve running performance (reducing total time), elevate serum glucose concentrations, decrease serum cortisol and insulin concentrations, and not affect the serum concentrations of GH and IGF-1. However, as shown in the results, this was not the behaviour of the analysed variables.
The main finding of this study was that carbohydrate supplementation during a simulated 13 km road race lasting between 45 and 65 min did not affect the athletes’ race time but induced significant changes in serum concentrations of GH, cortisol, insulin, and glucose.
Despite all the metabolic changes observed in this study, race time remained similar in the two situations studied, contrary to what is generally observed in the literature, which indicates that carbohydrate supplementation improves performance in endurance races [11].
In a recent meta-analysis, Ramos-Campo et al. [11] showed that, in most of the studies analysed, carbohydrate supplementation during exercise results in a significant improvement in endurance performance compared to a control condition. However, the effect of carbohydrates on performance was greater in long-duration exercise than in shorter-duration exercise. In addition, carbohydrate intake has a greater effect on less-trained participants than on more-trained participants. In contrast, the magnitude of the change in performance due to carbohydrate intake was not affected by dosage, the type of ergometer used, the form of carbohydrate intake (drink, solid, or gel), and the type of carbohydrate (simple vs combined) [11]. Thus, a possible explanation for our findings could be related to the training status of the athletes, i.e., the simulated race was not intense enough for the supplementation to impact the performance of experienced runners, or it was not long enough.
Studies showing a decrease in serum concentrations of GH and IGF-1 (suppression of the GH/IGF-1 axis), insulin, and glucose after intense physical exercise have linked this decrease to an increase in cytokines, which were associated with glycogen depletion. Therefore, glycogen deficiency is thought to be associated with an increase in the local expression of cytokines (IL-6 in muscle), a decrease in glucose transporters, an increase in cortisol, a decrease in insulin secretion, and β-adrenergic stimulation [8, 9]. Although cytokines were not measured in the present study, the GH/IGF-1 axis did not behave as expected. No significant differences in IGF-1 were observed in any situation, and serum GH levels increased after exercise, which also corroborates the hypothesis that the simulated race’s intensity or volume was insufficient to promote possible changes.
Monteiro et al. [4] monitored the kinetics of IGF-1 and IGFBP-3 in adult female bodybuilders and showed that, despite the resistance training performed by the athletes, the consumption of dietary supplements, and the use of anabolic steroids, there was a significant reduction in serum concentrations of IGF-1 and IGFBP-3 at the end of the bodybuilders’ preparation [4]. According to the authors, the calorie restriction used at the end of the preparation may have been the main factor associated with the decrease in IGF-1 concentrations. Magraner and colleagues [3] also found a strong association between calorie restriction and suppression of the GH/IGF-1 system when they studied young military personnel during calorie-restricted training, which was also observed when military personnel practised strenuous operational training [3, 13].
The present study revealed an interesting contrast compared to the effects of calorie restriction on the GH/IGF-1 axis observed in the studies by Monteiro et al. [4] and Magraner et al. [3]. In both contexts, there was a significant impact on key hormonal pathways, though the outcomes on performance and hormone regulation diverged. Despite notable metabolic changes, including altered serum concentrations of GH, cortisol, insulin, and glucose, no significant improvement in race performance was observed. This finding contrasts with much of the existing literature that suggests carbohydrate supplementation typically improves endurance performance by stabilising blood glucose and delaying fatigue [14].
Tourinho et al. [15] evaluated nine Brazilian jiu- jitsu fighters before and immediately after a standard training session and found no significant changes in IGF-1 and IGFBP-3 concentrations. However, the IGF-1 levels measured at rest before the training session were already lower than expected for this sample (178 ± 55 ng/mL). For the authors, this suppression observed before the training session reflects the moment the wrestlers were going through, i.e., a period of intense training and strict calorie restriction to reduce weight in order to participate in a championship. In the present study, no caloric restriction was reported [15]. Regarding changes in body mass, there was a decrease in both situations, most likely related to the water loss that occurred during the simulated road race.
In a study of Crossfit practitioners conducted by Alípio et al. [16], no significant change in IGF-1 and IGFBP-3 concentrations was observed, despite the fact that the Crossfit session was classified as having an intensity between three (moderate) and nine (very difficult) on the subjective perception of exertion scale. According to the authors, the Crossfit practitioners were already accustomed to high-intensity training and had a balanced diet, which is perhaps why the GH/IGF-1 axis growth mediators were not significantly reduced [16].
The literature shows that the acute suppression of the GH/IGF system, glucose, and insulin observed in some sports is closely related to intense training combined with calorie restriction, especially in sports that use the glycolytic system as the predominant energy source for adenosine triphosphate (ATP) resynthesis [17]. In this sense, the predominantly aerobic metabolic nature of the exercise used in this study, a simulated road race, and the absence of calorie restriction may be a possible explanation for the behaviour of GH, IGF-1 and glucose, which did not show a decrease in their values, regardless of whether the athletes were supplemented with carbohydrate or not. On the other hand, supplementation caused insulin to behave differently, increasing in the supplemented athletes and decreasing in the placebo drinkers.
The changes to the variables analysed in this study (GH, IGF-1, cortisol, insulin and glucose) showed an important relationship. Indeed, GH and cortisol are hormones that act to protect the body against hypoglycemia in endurance tests [18, 19], and in the present study, both had increased concentrations in the two situations studied, indicating that they acted effectively during the simulated road race to preserve the body’s glucose levels, up to the final sprint of the race. On the other hand, insulin and glucose increased at the end of the exercise performed with carbohydrate supplementation. Meanwhile, insulin decreased, and blood glucose was maintained at the end of the race performed without carbohydrate supplementation, demonstrating that glucose concentrations are maintained during prolonged exercise thanks to the modulation of insulin levels, insulin sensitivity, and changes in glucose transporters, which occur during this situation [19].
The present study presents new insights into hormonal changes, such as cortisol and GH, in response to carbohydrate supplementation in adult runners. This work innovates by verifying that carbohydrate supplementation can induce significant changes in serum concentrations of GH, cortisol, insulin, and glucose without affecting athletes’ race time. Furthermore, the findings provide a more detailed understanding of supplementation’s interaction between these variables and insulin regulation after running.
In conclusion, an increase in GH and cortisol levels was observed after a 13km simulated road race regardless of carbohydrate supplementation. A decrease in insulin levels was noted in the non-supplemented situation, while a rise in insulin and glucose concentrations was observed during supplementation. It seems reasonable to suggest that these changes contributed to the maintenance of IGF-1 and the race time of the runners evaluated, regardless of the carbohydrate supplementation. Further studies should be conducted on cytokines and IGF-1 binding proteins, with particular attention to IGFBP-3 and IGFBP-1, which act as a biomodulator of IGF-1 activity.
Practical applications
The findings suggest that, while carbohydrate supplementation during a 13 km race induced significant metabolic changes (affecting GH, cortisol, insulin, and glucose levels), it did not necessarily lead to immediate performance improvements in short-duration endurance events. Therefore, athletes should prioritise balanced energy intake, particularly during prolonged periods of intense training, to avoid hormonal imbalances that may impair recovery and performance.