Introduction
Multiple sclerosis (MS) is an autoimmune illness of the central nervous system, currently affecting about 2.5% of the population worldwide. It is a multifaceted chronic illness with a wide range of symptoms [1]; 49–59% of patients with MS experience dizziness, including postural intolerance [2]. Approx. 75–82% of mild to moderately disabled patients with MS have balance difficulties [3].
It has been shown that one of the main predictors of patient independence is gait deviations. Approximately 85% of MS patients report gait abnormalities as their primary complaint. Early during the MS disease, gait abnormalities occur. Up to 55% of patients will need a walking aid within five years of diagnosis, and 20% will be wheelchair-dependent, which inspired us to perform this research work [4].
The vestibular system plays a significant role in postural control, and different components of the vestibular system, including cranial nerve VIII, vestibular nuclei, oculomotor tracts, medial longitudinal fasciculus, and cerebellum along the peripheral and central vestibular pathways, may be affected in MS [5]. Accordingly, exercises that can retrain the sensory mechanisms to present precise physical cues for the location and head and body movements are included in vestibular rehabilitation [6]. These exercises focus on utilising the vestibulo-ocular and cervico-ocular reflexes, along with somatosensory retraining for balance and gait, in the form of a vestibular physical therapy program [7]. Vestibular rehabilitation is based on the mechanisms of vestibular adaptation and replacement [8], which have been effectively used in the management of central and peripheral vestibular lesions [9].
A vestibular rehabilitation program can comprise custom-tailored exercises for each patient or comprehensive predetermined exercise protocols, such as the cawthorne cooksey exercises. Even when performed a long time after the acute flare-up. cawthorne cooksey exercises incorporate head and trunk motions sufficient to stimulate the vestibular system, enhance balance, and minimise vertigo and dizziness [10].
Cawthorne cooksey exercise protocol reduces the impact of vestibular dysfunction, which helps MS patients feel less dizzy, have better postural control, and activate their lower limb extensor muscles, all of which improve trunk kinetics and gait [11].
Vestibular rehabilitation is intended to modify the central nervous system to reduce vestibular input and offset the loss of the vestibulo-ocular and vestibulo-spinal reflexes. This happens because of cawthorne cooksey exercises and balance retraining, which may enhance the dynamic stability and variability of the entire body [12].
One of the most often reported MS symptoms that negatively affects quality of life is walking impairment. Among the many frequent symptoms and deficits associated with MS, such as fatigue, weakness, stiffness, ataxia, vertigo, and balance issues, gait impairment is a defining feature of the disease. The ability to walk is crucial for assessing the progression and rehabilitation of MS patients [13].
Thus, the present study is designed to investigate the efficacy of vestibular rehabilitation for trunk kinetic and kinematic parameters in patients with multiple sclerosis. The hypothesis of the current study suggested the null hypothesis that there is no statistically significant effect of vestibular rehabilitation on trunk kinetic and kinematic parameters in patients with multiple sclerosis.
Materials and methods
Study design and participants
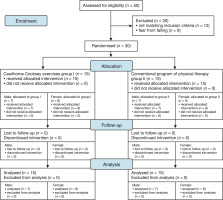
In this randomised controlled trial (Figure 1), we included patients with MS who were diagnosed and referred to our department by a neurologist. The following criteria were used for inclusion: (1) aged 35–55 years; (2) ambulatory without assistance; (3) diagnosed with relapsing-remitting MS by a neurologist, with approximately five relapse attacks of the hemiparesis type with a muscular tone of 1 or 1+ depending on the MAS, or modified Ashworth scale; (4) all individuals were evaluated by a neurosurgeon to confirm central vertigo using the Dix Hallpike manoeuvre [14]; and (5) degree of MS-related disability, based on the enlarged disability status scale, ranging from 2 to 2.5 (little disability) (EDSS) [15]. People with musculo-skeletal deformities, inner ear abnormalities, psychiatric disturbances, or seizures were excluded. All patients who satisfied the selection criteria and agreed to participate in the study signed a voluntary informed consent before participation.
Sample size
The sample size was determined using the G*POWER statistical program (version 3.1.9.2). Using an value of 0.05 to obtain 95% power (F tests, mixed design, repeated measures, within-between interaction), a target sample size of 30 subjects was computed.
Procedure
The two equal groups of patients were created (a double-blind, randomised controlled trial): group I exercised with cawthorne cooksey in addition to the conventional program of physical therapy, whereas group II only received the conventional program of physical therapy. Participants were randomly allocated through a secure system of opaque, closed envelopes. All patients in both groups received 12 sessions day-after-day, one hour per session and underwent two assessment sessions – before and after the physical therapy.
Interventions
The cawthorne cooksey exercises were administered as per the following protocol:
While lying in bed, head motions that start off slow and then quicken later on while the eyes are closed.
While seated, leaning over, and picking up objects from the ground while shrugging and circling their shoulders.
A little ball can be thrown from hand to hand (at eye level) while standing, as well as from sitting to standing while keeping the eyes open and closed. A ball can also be thrown from hand to hand under the knee while standing.
Moving around (in the treatment room) by crossing the room while keeping the eyes open and then closed, going up and down a slope while keeping the eyes open, and moving up and down steps while keeping the eyes closed.
Conventional physical therapy program (balance training): The following exercises were performed by both study groups for each session:
Kneeling balance in the forward and backward directions (repeat 20 times).
Side to side balance while kneeling (repeat 20 times).
Forward and backward weight shifting; this is accomplished by the patient rounded and arched down the back (repeat 20 times). Put the weight over the right hip first, then the left hip, while swaying from side to side. The hip should lift off the ground and the ribcage should oscillate (repeat 20 times).
Balancing while standing with both feet apart, then with both feet together, and with both eyes open and then both eyes closed (holding for 15 s for each).
Standing with a forward and backward lean (repeat 20 times).
Moving sideways and backwards on a workout step (repeat 20 times).
Standing on the unaffected leg and holding the position (holding for 5 s, repeat 20 times).
Outcome measurement
We used a Biodex isokinetic dynamometer (Model 2000, System 3 Pro: Biodex corporation, Shirley, NY, USA) for the kinetic and kinematic assessment of the trunk. An isokinetic dynamometer is an effective and trustworthy method to evaluate the kinetic and kinematic characteristics of the lumbar spine [16]. The dynamometer’s dual-position lumbar extension-flexion attachment was used with the patients in an upright position and hips and knees flexed at 90°. In relation to the anatomic reference position (0°), the trunk’s total range of motion was limited to 50°, with lumbar flexion set as 30° [17]. Hip flexion-extension movement was reduced by isolating the lumbar motion since only 50° trunk motion was allowed. Additionally, the application of a pad behind the sacrum, a strap on the pelvis, and the rotating axis of the dynamometer placed at the level of the anterior superior iliac spine reduced hip mobility during the test.
Fifteen successive maximal concentric lumbar flexion efforts in four sets were done with the trunk protocol done at a 60°/s angular velocity [18] with 1 min rest between sets.
Statistical analysis
The two groups’ average ages, weights, heights, and MMSEs were compared using descriptive statistics and an ANOVA test.
The sex distribution of the two groups was compared using the chi-square test.
The effects of time (before versus after), treatment (across groups), and their interactions on the average power, acceleration, and deceleration times of the trunk flexors were examined using a two-way mixed 3 × 2 MANOVA test.
All statistical tests had a significance threshold of p = 0.05.
The statistical package for social studies (SPSS) version 23 for Windows (IBM SPSS, USA, chicago, IL) was used to conduct all statistical tests.
Ethical approval
The research related to human use has complied with all the relevant national regulations and institutional policies, has followed the tenets of the Declaration of Helsinki, and has been approved by the physical therapy research ethics committee, cairo University, Egypt (approval No.: P.T.REc/012/003988) on 11/9/2022. clinical trial registration number (NcT05635890).
Results
We included a total of 30 MS patients in the study. The demographic and baseline Mini Mental State Examination (MMSE) data for all patients are presented in (Table 1). Both groups were comparable in terms of the average MMSE scores, height, weight, and patients (p > 0.05).
Table 1
Normative values of the demographic data of the two groups
Impacts of vestibular rehabilitation on the average trunk flexor power
The pre-treatment average power for the trunk flexors in groups I and II was 25.97 ± 6.66 W and 21.58 ± 3.67 W, respectively. On comparing the mean trunk flexor power in both groups, we found that there was no discernible difference between the two groups (p = 0.213) (Table 2).
Table 2
Normative values of trunk flexors’ average power of each group
After treatment, the average power of the trunk flexors in groups I and II were 51.93 ± 16.53 W and 26.3 ± 4.27 W, respectively. When compared, the average trunk flexor power of both groups indicated a substantial increase (p = 0.000) (Table 2).
Impacts of vestibular rehabilitation on the trunk flexor acceleration time
The mean and standard deviation (SD) values for the pre-intervention trunk flexor acceleration time were 108.7 ± 31.14 ms and 110 ± 39.1 ms, respectively. When comparing the mean between the two groups, there was no discernible difference (p = 0.213) (Table 3).
Table 3
Normative values of trunk flexors’ acceleration time of each group
After the intervention, the mean trunk flexor acceleration times were 58.67 ± 22.95 ms in group I and 90.67 ± 32.83 ms in group II. When compared with the pre-intervention value, the mean trunk flexor acceleration time significantly decreased in both groups (p = 0.000) (Table 3).
Impacts of vestibular rehabilitation on the trunk flexor deceleration time
At baseline, the mean value of the trunk flexor deceleration time was 311.33 ± 123.34 ms in group I and 379.33 ± 106.73 ms in group II. Regarding the pre-intervention values, there was no discernible difference between the two groups (p = 0.99) (Table 4).
Table 4
Normative values of trunk flexors’ deceleration time of each group
After the treatment, the mean trunk flexor deceleration time decreased to 170 ± 89.52 ms and 359.33 ± 106.73 ms in groups I and II, respectively, which was also statistically significant (p = 0.040) when compared (Table 4).
Discussion
Hoang [19] reported that in patients who performed the cawthorne cooksey exercises, the average trunk flexor power increased significantly more than other trunk kinetics. This considerable improvement can be linked to the vestibulo-spinal tract’s response to vestibular rehabilitation, which originates in the brain-stem’s lateral vestibular nucleus and travels to the trunk muscles’ spinal motoneurons in a single direction to control trunk power. Accordingly, it is possible that stimulation of the vestibular system may promote an increase in the tone of the trunk muscles, especially the flexors, resulting in the production of more energy.
Similar to our results, Fritz and Lusardi [20] also concluded that trunk flexor acceleration and deceleration time were significantly improved in patients who received cawthorne cooksey exercises. The authors suggested the role of controlled stimulation of the vestibular system, which improves trunk muscle power to facilitate trunk movement and decrease the flexor acceleration and deceleration time. Facilitation of trunk movements can further help in managing the imbalance that may arise from a disturbance in the vestibular systems caused by MS. The common symptoms of all vestibular diseases include vertigo, imbalance, and/or dizziness, and vestibular rehabilitation has been useful in alleviating these symptoms for a variety of vestibular illnesses [21].
It has been proposed that vestibular therapy promotes trunk dynamic stability through developing vestibular system stimulation and central compensation. As observed in our study, previous studies using vestibular therapy in patients with MS have also reported positive effects of the cawthorne cooksey exercises on dynamic trunk stability [22].
The study has certain limitations. First, some patients dropped out of the study due to the fear of falling and other logistic constraints. Second, due to the stringent inclusion criteria, we could only include a small number of cases of MS who had developed hemiparesis.
Conclusions
Our results reveal that the cawthorne cooksey exercises, when used along with conventional vestibular rehabilitation, result in greater improvement in trunk kinetic and kinematic parameters (average power, acceleration, and deceleration time) as compared to the conventional vestibular training strategies alone.