Introduction
Osteoarthritis (OA) is the most common kind of arthritis and the main cause of disability. Approximately 250 million individuals globally experience this degenerative and progressive joint disease [1]. Knee OA (KOA) is associated with impaired proprioception, pain, and dysfunction of the quadriceps, all of which can lead to impairment. Moreover, the ability of the quadriceps muscle to control force is compromised in individuals with KOA. It has been demonstrated that exercise therapy benefits KOA sufferers by lowering pain and enhancing their quality of life [2].
The rehabilitation of KOA is determined by many elements, such as the patient’s selection criteria, daily activities, age, the aetiology of the illness, and lesion grading [3]. Although total knee replacement has a high rate of success (81-90.3%), non-operative therapies or joint preservation procedures are still preferred over arthroplasty [4]. Platelet-rich plasma (PRP) injections, weight loss, muscle building, neuromuscular education, low-impact aerobic exercise, anti-inflammatory medicine [5], and pharmaceutical therapy are all non-surgical treatments for early OA. For KOA pain alleviation, pharmacological treatment is frequently ineffective [6]. Additionally, pharmacological drug use is frequently linked to significant adverse effects (AEs), such as haemorrhage and gastrointestinal ulcers [7]. KOA is also treated with complementary therapies, including local injections [8, 9], acupuncture [10, 11], moxibustion [12], cupping therapy [13], exercise [14], and laser therapy [15]. In recent years, extracorporeal shock wave therapy (ESWT) has become more widely accepted as a treatment for orthopaedic problems, including KOA [16]. Numerous musculoskeletal conditions have been treated by shock wave treatments.
The therapy has several effects, including pain alleviation, improved joint movement, and the prevention of avascular necrosis progression [17]. When compared to other treatments, shock wave therapy has various benefits, such as non-invasiveness, a reduced rate of complications, no hospitalisation, and reduced expense. As a result, shock wave treatment is often considered before surgery as an effective therapeutic option for several related disorders [18].
ESWT effects on tissues may be explained by several known plausible physical, physicochemical, chemical, and biological mechanisms. During the physical phase, a shockwave creates a positive pressure that permits energy to be absorbed, reflected, refracted, and transmitted to tissues. In the physicochemical phase, ESWT activates the pathways of focal adhesion kinase (FAK), extracellular signal-regulated kinase (ERK), and Tolllike receptor 3 (TLR3), to encourage cells to create biomolecules, including adenosine triphosphate (ATP) [19]. Shockwaves can mediate transmembrane cellular ion channels and intracellular calcium flux in the chemical phase. Therefore, enhanced angiogenesis, wound healing, non-union healing, modulation of tissue and nerve regeneration, and inhibition of inflammatory activities are just a few of the biological advantages of ESWT [20].
Iontophoresis is another approach for the treatment of KOA [21]. It has received a lot of attention in the last 25-30 years and has been utilised to manage common musculoskeletal diseases involving knee pain. However, questions remain in terms of its effectiveness in treating the symptoms of people with KOA [21, 22]. Iontophoresis is a therapeutic procedure in which ions are introduced into bodily tissues using a direct electrical current. Iontophoresis drug delivery has been reported as a viable alternative to hypodermic corticosteroid injections for KOA [23].
Strong analgesic, anti-inflammatory, and anti-swelling effects can be achieved with iontophoresis. It delivers medicinal ions of chemical compounds that are electrolytically dissociating under the influence of an electric field to reach deeper tissues through the skin or mucosa. Sweat glands are essentially the channel of least resistance through which ions from the substance enter the skin. It should be noted that one benefit of iontophoresis is that it can yield a high medication concentration in the target tissue without the need for oral administration, which reduces the risk of overdosage and negative effects [24]. Biological elements like the skin’s surface area, temperature, and local blood flow affect the effectiveness of the therapy [25].
Iontophoresis provides quick medication administration with few systemic adverse consequences. Furthermore, the pain associated with needle insertion in a region that is already tender can be avoided [22]. Avoiding the application of hypodermic needles also reduces infection risk and prevents further tissue harm [23]. Acetic acid, sodium salicylate, sodium diclofenac, benzydamine, ketamine, lidocaine, ketorolac, and naproxen have all been utilised, moreover hydrocortisone, dexamethasone, and magnesium sulphate [24].
The dexamethasone sodium phosphate (DEX-P) iontophoresis solution is an aqueous solution that is free of preservatives. It outperforms the preservative solution, which may include positively charged ions that compete with the adversely polarised dexameth-asone ions [21-25]. Recent research suggests that iontophoresis could be used to administer drugs without the need for needles or invasive penetration. A tiny electrical current is applied to force the ionically charged steroid medication into the skin, making it a transdermal technique for medication administration. Dexa-methasone through iontophoresis has been proven to be an efficient, non-invasive technique for lowering pain associated with knee injuries, including KOA [21-25]. Eighty percent of people diagnosed with unilateral KOA develop bilateral symptoms over 12 years, neuromuscular control and lower limb strength are compromised in individuals with OA in the knee and dynamic balance may raise the risk of falls [26]. To the best of our knowledge, there is still inadequate data to draw firm conclusions on which treatment is more effective for relieving pain and improving functional activity in individuals with KOA: clinical superiority of ESWT or dexamethasone iontophoresis. Thus, this pilot study aimed to assess and contrast the effectiveness of DEX-P iontophoresis and ESWT in treating individuals with unilateral symptomatic KOA.
Material and methods
Study design
The pre-post single-blind randomised experimental trial followed the principles of the Helsinki Declaration (1964) and the Consolidated Standards of Reporting Trials [26]. From March to the beginning of August 2021, this trial was held at the outpatient clinic of the Faculty of Physical Therapy. The protocol was acknowledged by the Faculty Research Ethics Commit tee (REDACTED) and documented at ClinicalTrials.gov (REDACTED). Informed written consent was obtained from the patients.
Subjects
Using the clinical criteria of the American College of rheumatology, 70 individuals of both sexes, aged 40 to 50, were diagnosed with unilateral symptomatic KOA at least three months earlier [27].
(1) Simple radiological pictures compatible with KOA, with Kelgren and Lawrence (K-L) grade 2 [28]; (2) pain intensity of 5 or above on the visual analogue scale (VAS) [29]; and (3) soreness on the medial tibial plateau [30] were the inclusion criteria.
The following were the exclusion criteria: (1) neurologic abnormalities or systemic illness; (2) intra-articular injections or surgical procedures during the previous six months; (3) any contraindication to MRI or radiography; and (4) a history of knee trauma [30]. Ten patients were excluded because they had received treatment in the last 3 months (Figure 1). Patients were chosen randomly from the outpatient clinic at the Faculty of Physical Therapy. Using an opaque lockable envelope, patients were assigned to one of three groups using a random generator 20. Quadriceps strengthening exercises and radial ESWT were administered to experimental group A (shock wave group). Quadriceps strengthening exercises and DEX-P iontophoresis 8 were administered to experimental group B (iontophoresis group). Only quadriceps strengthening activities were given to the control group (group C; strengthening group). The four-week therapy regimen was repeated twice a week. In this investigation, the patients were blinded to their group.
Sample size
The size of the sample was determined using the E-test (multivariate analysis of variance; MANOVA), MANOVA can identify patterns among the many dependent factors. Under MANOVA, it is possible to compare the group mean values and assess the effects of independent variables on the numerous dependent variables.
With a power of 80% and a Type I error of 5%. The effect size (0.42) was determined by the primary VAS results from a 15-subject pilot study used because VAS procedures are easier to complete and more straightforward than other approaches. The validity of VAS techniques is also well supported by empirical data, both in terms of test-retest and inter-rater reliability.
The minimum sample size was 45 and to account for dropouts, the number increased by 10%. Therefore, the appropriate sample size was 60. G* Power version 3.1.9.2 (Franz Faul, Uni Kiel, Germany) was used for the calculation.
Interventions
The entire sample received strengthening exercises. The shock wave group received radial ESWT, while the iontophoresis group received DEX-P iontophoresis. Shock wave and iontophoresis therapy was performed by an experienced therapist who followed the instructions and guidelines described in the literature.
Shock wave group (A)
The patient was placed in a supine position with 90 degrees of knee flexion, with the therapist standing next to the limb and tightly pressing the probe of the radial ESWT apparatus (EM12681015; EME Srl, Pesaro, Italy) on the most tender points at the medial tibial plateau level. Treatment was administered in a continuous motion with medium-energy radial ESWT (2000 shock/session [10 Hz]; EFD, 0.178 mJ/mm2). This was repeated twice a week for 4 weeks [30] (Figure 2).
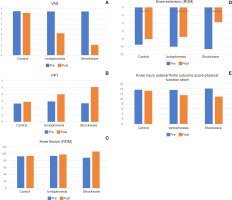
Figure 2
A – within and between group analysis for VAS (pain intensity), B – within and between group analysis for PPT (pain threshold), C – within and between group analysis for knee flexion range of motion, D – within and between group analysis for knee extension range of motion, E – within and between group analysis for KOOS-Ps (knee injury and osteoarthritis outcome score physical function short)
Iontophoresis group (B)
The iontophoresis group received 0.4% (DEX-P) iontophoresis using an iontophoretic drug delivery system (Phoresor® II auto model PM850; IOMED, Salt Lake City, UT, USA) with an amount of 100 mg/cm2 applied to the cathodic electrode with a syringe and the active electrode placed directly over the most painful points around the medial tibial plateau of the knee joint. The dispersive electrode was placed 6 inches away from the active electrode on the skin. If a patient reported any sensation other than tingling, the treatment was stopped, the electrodes were removed, and the skin was inspected. If the skin displayed signs of blistering, the session was discontinued and the patient was removed from the study. If the skin appeared normal, we continued the treatment at a lower current [31]. The device was programmed with the required dose (a 40-mA-minute dose) [21, 32-35], and the current intensity was gradually increased based on patient tolerance, ranging from 2 to 4 mA. The device calculated the time required for the chosen dosages automatically, and the therapy was performed twice weekly for a total of 4 weeks [12].
Control group (C)
A placebo-controlled trial is useful in determining whether an intervention has a specific effect [13]. Each patient completed three sets of 10 repetitions of a straight leg-raising exercise during each session. The patient started in a crock-lying position with one limb flexed. Then, the patient was permitted to elevate his or her limb to 45 degrees by contracting the quadriceps and holding for 6 s before gradually lowering the limb to the starting position and relaxing for 6 s [12]. Eventually, the patient did 30 repetitions over three sets of isometric quadriceps exercises [14]. The exercises were repeated twice a week for a total of 4 weeks.
Outcome measures
The patients were evaluated at the start of the study and again 4 weeks later after the treatment was completed.
Pain severity
VAS
A VAS was used to determine the severity of pain. The VAS was comprised of a straight line between two points: no pain or discomfort (0) at one end and the most excruciating pain that one could possibly feel (10) at the other end. This is a valid and reliable method for determining the severity of pain. To determine the level of pain, each patient was instructed to indicate their level of pain by placing a marker on the line [15].
Pressure pain threshold (PPT)
The Commander Algometer was used to determine the PPT (JTECH Medical, Midvale, UT, USA). It is a hand-held device that tests pain sensitivity in deeper structures by applying manual pressure. It is a valid and reliable pain assessment tool [16] that is frequently used. For measurement, the algometer’s tip was placed perpendicular to the skin around the most painful region of the knee, and the pressure was increased at a rate of 1 kg per second. The pressure was measured in kg/cm2 when the patient expressed discomfort and confirmed this verbally. The procedure was repeated three times at 60-second intervals with the average kg/cm2 value accepted as the PPT [17].
Knee function: Arabic-language version of the KOOS-PS
The Arabic-language version of the KOOS-PS is reliable and valid for measuring physical function in KOA [18]. The KOOS-PS comprises seven components, and the measure is assessed by adding the ranks of the seven items (or computing the average percentage). Scores of 0-7 (0-20%) indicate no physical function disability, scores of7-14 (20-40%) indicate mild physical function disability, scores of 14-21 (40-60%) indicate moderate physical function disability, scores of 21-28 (60-80%) indicate severe physical function disability, and scores of 28-35 (80-100%) indicate severe physical function disability [19].
Using the Knee Injury and Osteoarthritis Outcome Score, knee function was evaluated. The Physical Function Shortform (KOOS-PS) is a measure of physical function that is developed from the KOOS subscales related to sports and recreation and activities of daily living. Patients score how difficult it was for them to do the following activities over the course of the preceding week: (1) getting out of bed, (2) putting on socks or stockings, (3) getting up from a sitting position, (4) bending down, (5) twisting or pivoting on their affected knee, (6) kneeling, and (7) squatting [36].
Knee ROM
A digital electronic goniometer was used to measure passive knee ROM. This is a valid and reliable tool for the assessment of joint ROM. All measurements were made with a test person in a supine lying position. The hip tilt was at a fixed 20-degree angle. Calculating the maximum extension, the knee was first positioned at its greatest flexion and the other way around. The test subject was instructed to fully extend their leg after their ankle (35 x 20 x 7 cm) was placed on a resting block to measure their active maximum extension [19]. The digital goniometer was placed across the tibia’s ventral ridge to measure extension. Subsequently, the participant was instructed to extend their knee to its fullest extent while applying pressure with their hip on the resting surface. This was done to determine their active maximum flexion [37].
Statistical analysis
A normality test was performed on all of the data using a Shapiro-Wilk test. All data were normally distributed except for sex and the affected side. An ANOVA was utilised to analyse demographic data. The detection of the treatment effects and the interaction between time and treatment were analysed using a MANOVA. When differences between groups were observed, the Bonferroni test was used. The chi-square ^2) test was used to clarify the difference between groups in terms of sex and the affected side, and the partial eta square (η2) test was used to calculate the magnitude of differences between groups. SPSS version 23 (IBM Corp, Armonk, NY, USA) was used to conduct all analyses [36].
Results
Demographic data
According to the ANOVA, the three groups were matched in terms of demographic data. Demographic data of the 3 groups for age, weight, height, body mass index, sex distribution, and affected side (Table 1).
Table 1
Demographic data of the 3 groups for age, weight, height, body mass index, sex distribution and affected side
A general mixed MANOVA demonstrated a statistically significant difference between treatment groups with the following results: Wilks’ Lambda (X) = 0.122, f = 16.122, p = 0.0001, and η2 = 0.65. There was also a significant difference in terms of time: X = 0.029, f = 286.8, p = 0.0001, and η2 = 0.971. Finally, there was a significant interaction between treatment groups and time: X = 0.041, f = 33.64, p = 0.0001, and 2 = 0.797. In both the shock wave and iontophoresis groups, multiple pairwise comparisons using the Bonferroni test revealed statistically significant differences between all measures before and after treatment, with the significant differences favouring the ESWT group. There were no significant differences between the groups before therapy, but there was a significant difference between the iontophoresis group and the shockwave group after treatment. However, compared to the control group, there were no significant differences after treatment according to the Bonferroni test (Table2, Figure 2A-E).
Table 2
Within and between group analysis for VAS (pain intensity), PPT (pain threshold), knee flexion and extension range of motions, and KOOS-Ps (knee injury and osteoarthritis outcome score physical function short)
After the intervention, an improvement in VAS was identified in both groups. The comparison between groups showed a significant difference favouring the ESWT group, regarding the percentage of change in pain was 49.7% for the iontophoresis group and 76.3% for the ESWT group.
Regarding pain threshold percentages of change, the iontophoresis group had a 73.5% change compared to 86.4% for the ESWT group.
Regarding ROM, the improvement of flexion in the knee was identified in the ESWT group at 21.1% and iontophoresis group at 4.5%.
The improvement of extension in the knee was identified in the ESWT group at 64.28% and the iontophoresis group at 24.4%.
The improvement in the KOOS-Ps (knee injury and OA outcome score physical function short) was identified in the ESWT group as 29.8%, and the iontophoresis group was 15.4%.
Discussion
The current research showed a notable reduction in pain levels after shock wave application and iontophoresis in both treatment groups (A and B) compared to the control group, but the improvement in the ESWT (A) group was greater than that of the iontophoresis (B) group.
Dexamethasone, 4% iontophoresis, is a non-invasive physical therapy modality that allows direct control of inflammation in the underlying tissue. Reports have shown that adults with rheumatoid arthritis experienced a relief in knee pain after administration of dex-amethasone 4% iontophoresis [21]. Moreover, one report showed that it had a significant impact on lowering pain in patients diagnosed with medial collateral ligament (knee) sprain in comparison to the effect of dexamethasone with a placebo therapy [25].
When dexamethasone sodium phosphate and methylprednisolone sodium succinate were compared, dex-amethasone 4% sodium phosphate was found to be more stable than methylprednisolone sodium succinate. Dexamethasone 4% sodium phosphate can be stored at room temperature for 1 month without undergoing any changes. On the contrary, methylpred-nisolone sodium succinate should be used within 48 hours of mixing as the solution eventually loses its stability [38-41].
Dexamethasone iontophoresis can be effective in reducing epicondylitis symptoms such as pain, burning, or aching along the outside of the elbow and forearm. Patients who continue engaging in the actions that trigger the disease, can worsen and extend to the wrist. Deterioration was delayed as demonstrated in a 2003 study by Nirschl et al. [23], who discovered a 23-mm improvement in 100-mm VAS scores, in comparison to 14 mm for the placebo group, in addition to a better global advancement scale improvement (52% versus 33%). The first plausible reason for the improvement in the shock wave group could be hyper-stimulation analgesia. There are two main theories put forth describing the analgesia brought on by shockwave therapy. According to one of them, shockwaves cause tiny immunoreactive neurons in nerve fibres to deteriorate, thus lowering the number of mediators that promote inflammation. According to one theory, shockwaves activate the descending inhibitory system, which in turn releases endorphins and other analgesic chemicals, causing analgesia through hyperstimulation [42].
Overstimulation of the treated area could limit signal transmission to the brainstem, essentially shutting down the gate-control mechanism. The second possibility is that it acts on substance P, calcitonin gene-related peptide alpha (aCGRP) production in the dorsal root ganglion, and neurovascular sprouting to transmit information [24]. The vasoactive neuropeptide aCGRP increased nociception in primary OA of the knee, which has been demonstrated to impair joint integrity and cartilage in rheumatoid arthritis (RA) experiments. Not much is known regarding potential changes in a CGRP in the main articular structures involved in OA [41-43].
Shockwave therapy is generally regarded as safe and non-invasive, yet certain overstimulation symptoms are related to it, such as pain and discomfort. It is possible for patients to feel uncomfortable or in pain during or after the procedure. This is typically transient and goes away in a few days. There is a chance that the treated region will bruise or swell. This is usually a transient adverse effect that goes away in a few days to a week. Following therapy, some people may feel a brief increase in their level of discomfort. If any of the overstimulation issues that we asked our patients about should arise, we encouraged them to treat the region with a cold pack.
Not one of our patients experienced an overstimulation problem [43].
Shockwave therapy reduces pain, improves pinch test performance for at least six months, and reduces hand disability during the 6-month follow-up visit in patients with first carpometacarpal joint OA [44].
Some studies have demonstrated that when ESWT is utilised to heal rat KOA, it protects the damaged cartilage in addition to enhancing its healing [45-48]. In a clinical trial, ESWT was proven to have a significant impact on decreasing pain. According to research, ESWT could relieve pain by preventing the release of substance P. ESWT can inhibit the dorsal root ganglion’s production of a peptide linked to the calcitonin gene, which is linked to pain [25]. It could also act directly on peripheral sensory nerve endings, improving the pain threshold and preventing pain signal production and propagation.
This result is consistent with the findings of Speed’s research that concluded that an inflammatory response induced by growth factor secretion could help alleviate OA symptoms whilst also boosting angiogenesis, which can aid in repairing damaged tissues [25]. For instance, it was discovered that in a rat model of OA, 14 levels of neuropeptide calcitonin gene-related peptides were lowered in the dorsal root ganglia following ESWT therapy. It is believed that peptide 6 - which is represented by nociceptors - plays a part in the perception of joint pain [45].
Creaby et al. [26], found that throughout three sessions, group M, who had medium energy (0.093 mJ/mm2), had a lower pain score at one week and twelve weeks than group L, who had low energy (0.040 mJ/mm2). The threshold for local nerve excitation and signal transmission may be lowered in response to injury, inflammation, or other nociceptive stimuli [26]. This phenomenon, known as “peripheral sensitisation,” increases the responsiveness of peripheral nociceptors and may account for the phenomenon of hyperalgesia (or hypersensitivity) and allodynia (pain in response to normally no noxious stimuli) in some individuals with KOA, resulting in greater pain relief [27]. Unmyelinated C nerve fibres and myelinated A) fibres carry the action potential produced when nociceptors on peripheral nerve terminals are stimulated. Type C fibres respond to many impulses and cause a more diffuse burning pain feeling, whereas Αδ fibres transmit and experience intense pain due to their faster conduction [37].
From the joint, afferent pain fibres carry pain signals to the spinal cord, where they are processed synapti- cally. From there, they go along ascending routes to the thalamus and higher regions of the somatosensory cortex. The spine’s descending fibres assist in regulating the pain inputs. Numerous sociocultural and psychological elements, such as underlying anxiety and depression, have a significant impact on pain as well. The intricate interactions between these elements and the underlying neurologic architecture have given rise to the term “neuromatrix” [38].
The threshold for local nerve excitation and signal transmission may be lowered in response to injury, inflammation, or other nociceptive stimuli. This phenomenon, known as “peripheral sensitisation,” increases the responsiveness of peripheral nociceptors and may account for the phenomenon of hyperalgesia (or hypersensitivity) and allodynia (pain in response to normally nonnoxious stimuli) in some individuals with KOA [37].
The medium energy group showed greater improvements because the histological response to the ESWT is dose-dependent based on overall energy [27].
Lizis et al. [29] examined the 12-week efficacy of ESWT in patients with KOA in comparison to a placebo.
Over the course of the 12-week therapy session, they found that ESWT is helpful in lowering pain and increasing knee function. They found out that ESWT potentially improves knee function and reduces pain [29].
In a comparative study, the impact of kinesiotherapy and ESWT on the subjective health and range of motion (ROM) of the afflicted knee were investigated. When compared to kinesiotherapy on the afflicted knee, ESWT was reported to significantly reduce pain, physical function, and ROM in individuals with KOA. Five shock wave sessions were administered to twenty individuals in the ESWT, one per week [30].
Hou et al. [18] Examined the safety and effectiveness of radial ESWT in Brazilians suffering from severe pain from primary KOA, in contrast to the present findings. Results [19] showed that radial ESWT was not effective in treating patients with severe pain from primary KOA. A research study of 38 individuals with KOA found that changes in pain were directly correlated with changes in muscle strength and proprioceptive acuity from exercise.
The study also examined the correlations between pain, proprioception, and strength before and after an 8-week home exercise program.
The electrical repulsion of the ionised drug from the electrode and the drug’s solvent-mediated electro-osmosis into the stratum corneum are the mechanisms responsible for the advances in the iontophoresis group [18]. This also improves patient compliance through less frequent dosages and capacity to customise medication treatment at predetermined rates according to specific needs [26, 49].
The outcomes of traditional Chinese exercise were shown to be more successful in reducing KOA symptoms than quadriceps strengthening exercises, with no significant difference observed between the control group before and after therapy [41].
This study was limited by its lack of follow-up, which hindered our capacity to look into how the patients changed over time. Consequently, more research will be required to evaluate the long-term impact of varying therapy duration on pain and function in KOA patients.
Conclusions
The finding of this study was that shock wave treatment in patients with KOA was superior to iontophoresis. The knee flexion and extension ROM, the pain threshold (PPT), the KOOS-Ps, and the knee flexion and extension scores were the outcome measurements.
Implications of physiotherapy practice
In clinical practice, ESWT is recommended because it is more effective than DEX-P iontophoresis in reducing pain and improving ROM in KOA patients. ESWT is effective in KOA; however, further research, including several samples is required to fully assess the treatment’s effectiveness.