Introduction
A syndrome in medicine usually consists of at least three interconnected symptoms that always occur together, but there can be more [1]. The global prevalence of metabolic syndrome has increased significantly in recent years, posing a major public health concern due to its association with higher risks of cardiovascular diseases, type 2 diabetes and overall mortality [2]. As the global population ages, the prevalence of metabolic syndrome among older individuals continues to rise, with approximately one in three adults over the age of 60 affected by this cluster of risk factors, including abdominal obesity, hypertension, dyslipidemia, and insulin resistance [3]. These conditions are particularly concerning in middle-aged and older adults due to the compounded effects of ageing, such as reduced physical activity, muscle mass loss, and changes in metabolic function. While factors such as genetics, diet and lifestyle also contribute to the onset of metabolic syndrome, physical activity has emerged as one of the most effective interventions for both prevention and syndrome management.
In the treatment of many diseases, physical activity is used alongside adequate dosing, and as a preventative of metabolic syndrome, it depends on the population and region [4], patient’s current health [5] and the effects contributing to physical activity [6]. Although the most common causes of metabolic syndrome are insufficient physical activity, lack of time [7], poor diet, and bad life habits (chronic stress, smoking, alcohol consumption) [8], people who are physically active for at least 30 min several times a week have a significantly lower risk of developing metabolic syndrome [9]. Another accompanying factor and component for the development of metabolic syndrome is obesity, which is associated with increased blood pressure, glucose, waist circumference, lipid levels, inflammatory markers, insulin resistance, oxidative stress, etc. In the abovementioned population, regular physical activity has also been linked to improvements in physical function, mental health, and quality of life [10]. Despite these benefits, older individuals often face barriers to engaging in physical exercise, including physical limitations, comorbidities, and lack of motivation. However, recent studies have highlighted that tailored physical education programs, even at moderate intensities, can lead to significant health improvements and help mitigate the risk factors of metabolic syndrome in this age group [11–13].
Based on recent studies, preventing metabolic syndrome through exercise today can result in a prevalence reduction ofapproximately 15–30% in older adults, depending on the type of exercise and the duration of interventions [14, 15]. Advantages and treatment with moderate intensity such as walking 4–5 km every day has positive effects on waist circumference (–5 cm), body weight (–3 kg), blood glucose (–20%), cardiovascular risk (–15%), blood pressure (–10 mm Hg), mood, quality of life and self-confidence [16]. In contrast, the study [6] is not consistent with other studies because the results show that moderate-intensity exercises, such as walking and cycling, are not effective in preventing metabolic syndrome in the elderly (50-69 years old) and that moderate intensity has no significant effects. In a comparison of aerobic interval training and strength training regimes in the treatment of metabolic syndrome, both types of training showed positive results, but aerobic interval training was found to be more effective [17]. Recent studies suggest that the effectiveness of exercise programs for improving insulin resistance is influenced by both the duration and intensity. For example, a study highlights that moderate- to high-intensity exercise lasting at least 150 min per week is beneficial for insulin sensitivity [18]. Similarly, a meta-analysis recommends incorporating both aerobic and resistance training into exercise regimens to optimally improve insulin resistance, with the total weekly exercise duration often exceeding 150 min [19]. These findings align with the idea that more substantial exercise volumes can enhance metabolic outcomes compared to shorter durations. By analysing and using data from an insulin resistance study, the WHO has again noted a higher prevalence of metabolic syndrome in men, in some cases even twice has high or more than in women [20]. Recent data indicate that the prevalence of metabolic syndrome has increased compared to earlier studies. According to the American Heart Association 2030 Impact Goal – A Presidential Advisory from the American Heart Association, metabolic syndrome is now a significant concern, with the prevalence varying by region [21]. Demographically, the prevalence ranges between 20% and 30% of the adult population, depending on the country in Europe [22] or Asia [23]. Global data underscore the increasing prevalence of metabolic syndrome and its implications for public health [24]. The prevalence of metabolic syndrome also increased with age in both sexes. Regarding age, this study shows that metabolic syndrome is more common in women (42%) than in men (24%). The lowest prevalence was recorded in the male age group 20–29 years (9.8%), followed by 30–39 years (16.3%), 40–49 years (25.5%), 50–59 years (33, 9%), 60–69 years (37.9%) and > 70 years (34.5%). For females, the lowest prevalence was recorded in the age group 20–29 (9.3%), followed by 30–39 years (24.3%), 40–49 years (48.3%), 50–59 years (64.1%), 60–69 years (67.2%) and those older than 70 years (67.1%) with metabolic syndrome [25].
Metabolic syndrome represents a global public health problem, and physical activity has been shown to be a key factor in its prevention and treatment. Given the high prevalence of metabolic syndrome, especially in the population over the age of 40, and the differences in the effects of various types of physical activity, there is a need for further research to better understand which physical activities most effectively influence the parameters of metabolic syndrome. Additionally, while evidence suggests that physical activity can reduce the risk of developing metabolic syndrome, the specific effects of different exercise programs have not yet been fully clarified, particularly regarding the relationship between exercise intensity, duration, and health outcomes. Therefore, the aim of this study was to determine, based on a summary of relevant literature, whether individual physical activity programs affect metabolic syndrome parameters in middle-aged and older adults.
Material and methods
Literature identification
A literature search was conducted following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines [26, 27]. Studies were sourced from databases such as Google Scholar, PubMed, Mendeley, Science Direct, and Scopus, covering research from 2000 to 2025. Keywords included ‘metabolic syndrome’, ‘aerobic exercise’, ‘resistance training’, ‘HIIT’, ‘SIT’, ‘inflammatory markers’, ‘middle-aged’ and ‘older adults’.
All relevant studies were screened for inclusion based on their alignment with the study objectives. Titles, abstracts, and full texts were reviewed by two independent authors. Any disagreements regarding study inclusion were resolved through discussion or consultation with a third reviewer to ensure accurate selection.
Inclusion criteria
Studies included in this review were those conducted between 2000 and 2025, published in English in full-text, with participants aged 40 years and above. All participants had to be middle-aged and older adults, whereas each study had to employ exercise protocols focused on improving markers related to metabolic syndrome.
To evaluate the effects of various types of physical activity on metabolic syndrome markers, a set of standardised protocols was applied. The analysed studies used different exercise interventions, including aerobic exercise (AE), resistance training (RT), high-intensity interval training (HIIT) and sprint interval training (SIT). These protocols were designed to assess parameters such as inflammatory markers (CRP, TNF-n, IL-6, IL-8), lipid profiles (LDL, HDL, TG), glucose and insulin levels, blood pressure (SBP, DBP) and aerobic capacity (VO2max).
Exclusion criteria
Studies conducted before 2000, published in languages other than English, involving participants younger than 40, or those with participants without the diagnosed metabolic syndrome were excluded. Studies without detailed methodologies on the exercise protocols used or without an assessment of metabolic syndrome markers were also not considered.
Risk of bias assessment
The risk of bias was assessed using the PEDro scale to determine the quality and potential biases in the reviewed studies [28]. Two independent reviewers assessed each study, calculating the k-statistics to estimate concordance. In cases of disagreement, a third reviewer provided a final assessment.
Data extraction
Following a cross-examination process, data were extracted and organised into an Excel spreadsheet. Extracted information included study characteristics such as the following: author, publication year, sample size, participant age, intervention type, duration, frequency, and study outcomes. The Cochrane Consumer and Communication Review Group’s standardised data extraction protocol was used to ensure uniformity.
Results
Included study quality
Assessment scores were ultimately determined based on the total number of included studies and the points each piece of research received on the PEDro scale. It has been determined that scores between 8 and 11 represent the ideal range. Research is categorised as ‘poor’ quality if it receives 0–3 points, ‘fair’ quality for 4–5 points, ‘good’ quality for 6–8 points, and ‘excellent’ quality for 9–10 points. Consequently, all included studies demonstrated overall good quality, as presented in Table 1 [29] (see before the references).
Study selection and characteristics
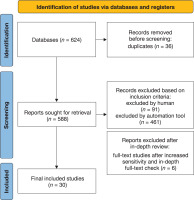
A total of 624 studies were found by searching databases and reviewing the studies’ reference lists. At first, a total of 36 studies were eliminated after the duplicates were reviewed. With regard to the inclusion criteria, 552 studies were excluded. From that point, a total of 36 studies were sought for retrieval, where an additional 6 studies were excluded (after an in-depth check). Ultimately, the systematic review consisted of a total of 30 full-text studies (Figure 1).
Table 2 (see before the references) resents the effects of various forms of exercise on inflammatory markers across multiple studies. These 30 studies included participants aged between 39.6 and 73 years from various countries, such as South Korea, Japan, the USA, Slovenia, Brazil, Saudi Arabia, Italy, Australia, Iran, Greece, China, Lithuania, Bulgaria, the Netherlands, Spain, and Finland. The total number of participants was 2,962, of which 971 were men and 1,991 were women. Sample sizes ranged from 24 participants [30] to 790 participants [31]. The interventions lasted from 2 weeks [32] to 52 weeks [33]. Different forms of physical activity were applied in the studies, including aerobic training [34–46], high-intensity interval training (HIIT) [47, 48], resistance training (RT) [49–51], combined aerobic and resistance training [33, 52–54], sprint interval training (SIT) [55, 56], and neural resistance training with aerobic intervals [57]. Training frequency typically ranged between 2 to 5 sessions per week [36, 42, 43], with session durations most commonly lasting between 30 and 90 min [37, 38, 44]. Exercise intensities were generally prescribed at moderate to high levels, with aerobic training performed at 50–85% of maximal oxygen uptake (VO2max) or heart rate reserve (HRR) [37, 44, 51], and resistance training commonly structured with 2–3 sets per session [51]. Some studies implemented combined aerobic and resistance training protocols, often involving approximately 40 min of aerobic exercise followed by 20 min of resistance training per session [33]. High-intensity interventions, such as sprint interval training (SIT), typically involved repeated sprint efforts interspersed with recovery periods [56], while others focused on maintaining exercise at lactate threshold intensity [55]. Such variation in training prescriptions reflects the diverse approaches to optimising improvements in inflammatory markers and metabolic health in middle-aged and older adults. The variables used in the studies include inflammatory markers such as TN F-α, IL-6, CRP, IL-8, IL-10, and IL-18 [33, 37–39, 43, 44, 49, 50, 52, 56–58]. Lipid parameters, including HDL, LDL, and triglycerides, were also analysed [3, 30, 34, 35, 46, 59, 60]. Glucose and insulin were measured variables in certain studies [3, 36]. Systolic and diastolic blood pressure were also frequently analysed [30, 34, 46, 59], while aerobic capacities, such as VO2max and VO2peak, were assessed to evaluate cardiovascular fitness [32, 59–61]. These studies consistently demonstrate the positive effects of physical activity on these health markers, highlighting significant improvements across multiple interventions. A reduction in C-reactive protein (CRP) levels was observed in 56.7% of the studies, while a decrease in interleukin-6 (IL-6) was reported in 40.0% of the studies, and a reduction in tumour necrosis factor-alpha (TNF-α) in 43.3%. An increase in interleukin-10 (IL-10) levels was noted in 13.3% of the studies. Regarding the lipid profile, an increase in HDL cholesterol concentration was reported in 23.3% of the studies, a reduction in LDL cholesterol in 10.0%, and a decrease in triglycerides in 13.3%. An improvement in aerobic capacity (VO2max or VO2peak) was reported in 13.3% of the studies, further confirming the positive effects of physical activity on endurance. A reduction in systolic and diastolic blood pressure (SBP and DBP) was observed in 16.7% of the studies, while a decrease in waist circumference (WC) was recorded in 3.3% of the studies. Improvements in glyce-mic regulation, including reductions in HbA1c, glucose (GLU), and insulin (INS) levels, were reported in 6.7% of the studies. Additionally, reductions in other inflammatory markers, such as interleukin-8 (IL-8) in 10.0% and interleukin-18 (IL-18) in 6.7% of the studies, were also recorded.
Discussion
Physical activity plays a key role in the prevention and treatment of many diseases associated with ageing, which has been confirmed in research [62]. In addition, regular exercise has shown positive effects on the metabolie syndrome (MetS), which is defined by the presence of at least three of the five major risk factors for cardiovascular disease: elevated blood pressure, diabetes, hypercholesterolemia, visceral obesity, and low HDL-eholesterol [62–64]. However, despite extensive research, there are still some knowledge gaps and inconsistencies regarding the impact of different exercise types and intensities on MetS-related outcomes [66].
This study investigates the effects of different exercise regimens on components of the metabolic syndrome (MetS), inflammation, and cardiometabolic risks, with a particular focus on aerobic and combination programs. Our analysis included a wide range of studies that looked at different exercise interventions, such as aerobic training, strength training, high-intensity interval training (HIIT), and combined aerobic and resistance training, on changes in inflammatory markers and other biomarkers of MetS. In conclusion, the results show a significant effect of exercise on reducing systemic inflammation, improving lipid profile, reducing body fat, and improving cardiovascular function, which is crucial for the management of MetS.
Aerobic training was highly effective in reducing markers of inflammation and improving metabolic parameters in individuals with the metabolic syndrome (MetS), including reductions in systolic and mean blood pressure, as well as improvements in blood lipids and glucose levels [67, 68]. In addition, aerobic training led to an increase in anti-inflammatory cytokines, such as interleukin 10 (IL-10), indicating a beneficial effect on inflammatory processes. Combined aerobic and resistance training, however, showed even greater benefits, because in addition to reducing inflammation markers and improving metabolic parameters, it also led to a significant increase in muscle strength, improvement in functional capacity (such as the results of the standing up from a chair test and grip strength), as well as perceptions of physical capacity, which together contribute to a significant improvement in the quality of life of people with MetS [44].
Increased arterial stiffness is considered an indicator of premature vascular ageing [69]. In the MetS group, the initial biological vascular age was 6 years older than in healthy individuals. The reduction in cfPWV in MetS subjects with aerobic training corresponded to a 5-year reduction in age-related arterial stiffness, which reduced the Framingham Risk Score (FRS) for cardiovascular disease by 4% [70]. Also, a reduction in cfPWV was associated with a 6 mm Hg reduction in central systolic blood pressure (cSBP), which has clinical significance, as a 2 mm Hg reduction in cSBP reduces cardiovascular mortality by 7% [70]. Aerobic training did not lead to changes in the carotid intima-medial layer thickness (cIMT) or other parameters of arterial dynamics in MetS subjects, which is similar to results in healthy men [71, 72]. Aerobic training, combined with weight loss, has been shown to significantly reduce C-reactive protein (CRP) levels and lower the risk of developing cardiovascular disease [31, 59]. The results of these studies align with previous findings showing that aerobic training significantly reduces TNF-α, a key pro-inflammatory cytokine [39]. Also, aerobic training has been shown to positively affect endothelial function [73], and it can also improve a comprehensive metabolic profile including insulin sensitivity and lipid levels, which is critical for the management of metabolic syndrome [60]. There are contradictory results in research dealing with the influence of aerobic intensity and volume on insulin sensitivity and other metabolic parameters. Some studies suggest that higher doses of aerobic training (> 2000 kcal/week) are required to improve insulin sensitivity and β-cell function in prediabetic adults [74]. Although high-intensity aerobic training has been shown to improve insulin sensitivity more than low-or moderate-intensity training when total training volume is held constant [36, 75], other research suggests that moderate-intensity aerobic training may be more effective in improving insulin sensitivity when total energy expenditure is controlled [76]. For example, long-term aerobic training at high intensity (80% VO2peak) has been found to provide greater benefits for insulin action compared to moderate (65% VO2peak) or low intensity (50% VO2peak) training [36]. In contrast, another study, by McGarrah et al. [76], found that moderate-intensity aerobic training improved insulin sensitivity more than high-intensity aerobic training, possibly via better fatty acid metabolism in skeletal muscle [77]. Also, a recent randomised controlled trial (RCT) comparing three combined aerobic and resistance training sessions of varying aerobic intensities over 12 weeks showed that insulin sensitivity was most significantly improved in the moderate-intensity group [76]. However, the amount of resistance training required to improve markers of glucose metabolism remains unclear, especially when combined with aerobic training [78]. Another randomised controlled trial (RCT) examined the effect of different volumes of resistance training (low volume: 3 sets/exercise compared to high volume: 6 sets/exercise) at the same intensity (70% 1RM), adding to the ongoing debate about the optimal training structure [78].
HIIT has become popular due to its effective and rapid effects on reducing components of MetS [79–82]. In the studies, high-intensity interval training showed a significant effect on reducing body fat, improving endothelial cell function, and reducing levels of inflammatory markers [66, 32]. Additionally, research confirms that HIIT leads to improved cardiovascular function and reduced inflammation, and that the effects of HIIT are comparable to those induced by continuous aerobic training [60]. The level of IL-6, an anti-inflammatory cytokine secreted during exercise, is increased in response to HIIT, which may act as a protection against systemic inflammation [83]. In the studies, HIIT also improved vascular function and reduced blood lipid levels [41, 57]. On the other hand, research on the effects of strength training and combined programs indicates similar benefits, but also certain differences. One study found no significant changes in endocrine markers such as insulin-like growth factor 1, cortisol or insulin profile after a 12-week strength training program (3 times per week, 16–18 RM to 8–10 RM) compared to continuous aerobic exercise cycling (1630 min at 60–90% HRmax) in older women [81]. Similarly, [80] reported no changes in blood cholesterol and glucose levels after 12 weeks of combined strength training and walking. An extensive study did not observe improvements in insulin sensitivity after 12 weeks of a combined RT+MICT program [79], although a metaanalysis and a long-term study reported significant benefits from longer interventions [38, 84]. Namely, RT+MICT over 3–13 months reduces the systolic blood pressure and waist circumference and increases HDL-C. Long-term programs have also been found to reduce HbA1c, hs-CRP, and HOMA-IR, but without significant effects on LDL-C [33].
Combined aerobic and resistance training has been shown to be the most effective for reducing inflammation and improving metabolic parameters. Such interventions reduce the levels of pro-inflammatory cytokines like TNF-α, IL-6, and IL-8, while simultaneously increasing the levels of IGF-1, which is associated with reduced body fat and improved insulin sensitivity [52, 55]. These effects are supported by the findings of the study [33], which indicated a significant effect of exercise on reducing pro-inflammatory cytokines and improving glycaemia in individuals with type 2 diabetes and MetS. The results also emphasise the importance of combined training in reducing visceral fat and improving vascular function, which is crucial for preventing cardiovascular diseases [42, 53].
Resistance training, although often overlooked in the context of MetS, also has a significant impact on reducing inflammatory markers and improving metabolic functions. Resistance training showed a positive effect on reducing TNF-α, IL-6, and CRP, as well as improving insulin sensitivity and muscle strength [49, 50]. Studies found that resistance bouts, especially when applied in combination with aerobic training, can significantly improve the lipid profile and reduce body mass, thereby reducing the risk of cardiovascular disease and type 2 diabetes [32, 40].
Importance of inflammation in metabolic syndrome
Inflammation is a key component of MetS and its reduction is of great importance in the prevention of cardiovascular disease. In this context, exercise training plays a key role. The results indicate that exercise reduces pro-inflammatory cytokines such as IL-6 and TNF-α, while simultaneously increasing anti-inflammatory cytokines like IL-10, which play an important role in reducing systemic inflammation [39, 40, 60]. These results confirm findings that suggest the effects of exercise on inflammation are independent of weight loss, indicating that exercise has an immediate impact on reducing inflammation and improving metabolic health [33].
Limitations and future perspectives
Although these findings provide significant support for the inclusion of exercise in therapeutic protocols for the management of MetS, it is important to note several limitations of the studies used in this analysis. First, many studies have relatively small samples, which may limit the generalisability of the results. Another challenge is the heterogeneity of research, in terms of different exercise regimes, durations of the interventions and specific populations involved. Based on this, future studies should focus on larger and more diverse samples, to confirm the effects of different exercise regimens on systemic inflammation, and to investigate how individual characteristics, such as age, sex, and baseline physical fitness, may modulate these effects.
Conclusions
Given the many benefits that exercise offers in terms of reducing inflammation and improving cardiovascular health, it is clear that exercise should be a key component of therapy for people with MetS. Aerobic, resistance, HIIT, and combined bouts show significant changes in inflammatory markers, metabolism, and vascular function. Further research should investigate optimal exercise protocols for different patient subgroups, in order to maximise the therapeutic effects of exercise in the context of MetS.
Table 1
PEDro scale results
| Study | Criterion | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | Total | |
| [61] | ✓ | ✓ | ✓ | × | × | ✓ | ✓ | ✓ | ✓ | ✓ | 7/10 |
| [35] | ✓ | ✓ | ✓ | × | × | ✓ | ✓ | × | ✓ | ✓ | 6/10 |
| [36] | ✓ | ✓ | ✓ | × | × | ✓ | ✓ | × | ✓ | ✓ | 6/10 |
| [39] | ✓ | ✓ | ✓ | × | × | ✓ | ✓ | × | ✓ | ✓ | 6/10 |
| [59] | ✓ | ✓ | ✓ | × | × | ✓ | ✓ | ✓ | ✓ | ✓ | 7/10 |
| [50] | × | × | ✓ | × | × | ✓ | ✓ | ✓ | ✓ | ✓ | 6/10 |
| [33] | ✓ | ✓ | ✓ | × | × | ✓ | ✓ | × | ✓ | ✓ | 7/10 |
| [40] | ✓ | ✓ | ✓ | × | × | ✓ | ✓ | × | ✓ | ✓ | 6/10 |
| [34] | ✓ | ✓ | ✓ | × | × | ✓ | ✓ | × | ✓ | ✓ | 6/10 |
| [55] | ✓ | ✓ | ✓ | × | × | ✓ | ✓ | × | ✓ | ✓ | 6/10 |
| [46] | ✓ | × | ✓ | × | × | ✓ | ✓ | ✓ | ✓ | ✓ | 6/10 |
| [30] | ✓ | × | ✓ | × | × | ✓ | ✓ | ✓ | ✓ | ✓ | 6/10 |
| [51] | ✓ | ✓ | ✓ | × | × | ✓ | ✓ | ✓ | ✓ | ✓ | 7/10 |
| [54] | ✓ | ✓ | ✓ | × | × | ✓ | ✓ | ✓ | ✓ | ✓ | 7/10 |
| [53] | ✓ | ✓ | ✓ | × | × | ✓ | ✓ | × | ✓ | ✓ | 6/10 |
| [58] | ✓ | ✓ | ✓ | × | × | ✓ | ✓ | ✓ | ✓ | ✓ | 7/10 |
| [44] | ✓ | ✓ | ✓ | × | × | ✓ | ✓ | ✓ | ✓ | ✓ | 7/10 |
| [37] | ✓ | ✓ | ✓ | × | × | ✓ | ✓ | ✓ | ✓ | ✓ | 7/10 |
| [42] | ✓ | × | ✓ | × | × | ✓ | ✓ | ✓ | ✓ | ✓ | 6/10 |
| [38] | ✓ | ✓ | ✓ | × | × | ✓ | ✓ | ✓ | ✓ | ✓ | 7/10 |
| [56] | ✓ | × | ✓ | × | × | ✓ | ✓ | ✓ | ✓ | ✓ | 6/10 |
| [57] | ✓ | ✓ | ✓ | × | × | ✓ | ✓ | ✓ | ✓ | ✓ | 7/10 |
| [52] | ✓ | × | ✓ | × | × | ✓ | ✓ | ✓ | ✓ | ✓ | 6/10 |
| [41] | ✓ | ✓ | ✓ | × | × | ✓ | ✓ | × | ✓ | ✓ | 6/10 |
| [45] | ✓ | ✓ | ✓ | × | × | ✓ | ✓ | × | ✓ | ✓ | 6/10 |
| [43] | ✓ | ✓ | ✓ | × | × | ✓ | ✓ | × | ✓ | ✓ | 6/10 |
| [49] | ✓ | ✓ | ✓ | × | × | ✓ | ✓ | ✓ | ✓ | ✓ | 7/10 |
| [66] | ✓ | × | ✓ | × | × | ✓ | ✓ | ✓ | ✓ | ✓ | 6/10 |
| [32] | ✓ | ✓ | ✓ | × | × | ✓ | ✓ | ✓ | ✓ | ✓ | 7/10 |
[i] 1 – eligibility criteria specified, 2 – random allocation, 3 – concealed allocation, 4 – groups similar at baseline, 5 – blinding of subjects, 6 – blinding of therapists, 7 – blinding of assessors, 8 – adequate follow-up (> 80%), 9 – intention-to-treat analysis, 10 – between-group comparisons, 11 – point measures and variability reported
✓ – criterion is satisfied, × – criterion is not satisfied
Table 2
Included studies
| Study | Country | Participant sample | Average age | Exercise mode | Intervention details | Biomarkers (results) |
|---|---|---|---|---|---|---|
| [61] | Slovenia | M – 30 | 50 | HI, CE | 12 weeks, 3 × week | VO2max ↑ |
| [35] | USA | n – 159 M – 89 F – 70 | 52.5 | HI and LI, cycling, treadmill | 6 months | LDL ↓ HDL ↑ |
| [36] | USA | F – 27 | 73 | LI, MI, HI, AE | 9 months, 4 × week | INS ↓ GLU ↓ |
| [59] | Japan | F – 459 | 49.5 | MI and HI, AD, stairs | 14 weeks, 3 × week | SBP ↓ DBP ↓, HDL ↑, TG ↓, VO2max ↑ |
| [39] | Greece | n – 60 M – 30 F – 30 | 61.6 | AE | 24 weeks, 4 × week | CRP ↓, TNF-α ↓, IL-18 ↓, IL-10 ↑ |
| [50] | Australia | n – 30 M – 15 F – 15 | 50.8 | RT | 10 weeks, 3 × week | CRP ↓, IL-8 ↓, IL-6 ↓, TNF-α ↓ |
| [40] | Greece | n – 47 M – 23 F – 24 | 58.5 | AE | 16 weeks, 4 × week | CRP ↓ |
| [34] | South Korea | F – 790 | 40 | LI, MI, HI, RA | 6 months, 3 × week | WC ↓, SBP ↓, DBP ↓, HDL ↑ |
| [33] | Italy | n – 150 M – 75 F – 75 | 62.1 | AE, AE+RT | 52 weeks, AE: 2 × week; AE + RT: 40 min AE + 20 min RT | IL-6 ↓, CRP ↓, TNF-α ↓, IL-10 ↑ |
| [46] | South Korea | F – 43 | 52.5 | LI, MI, AE | 16 weeks | SBP ↓, DBP ↓, TG ↓, HDL ↑ |
| [30] | The Netherlands | n – 24 M – 12 F – 12 | 52.5 | MI BE | 5 × week, 8 weeks | HbA1c ↓, HDL ↑, SBP ↓, DBP ↓ |
| [55] | Brazil | n – 48 M – 24 F – 24 | 53.9 | AE, AE+RT | cycling at lactate threshold | IL-6 ↓ |
| [51] | USA | n – 204 M – 120 F – 84 | 57.3 | AE, RT, AE+RT | AE: 50–80% VO2max; RT: 2–3 sets; AE + RT combined | CRP ↓ |
| [53] | Bulgaria | n – 79 M – 40 F – 39 | 47.5 | AE+RT | 24 weeks, 2–4 × week | CRP ↓ |
| [58] | Finland | n – 115 M – 75 F – 40 | 54.3 | AE, RT | 12 weeks, AE: 3 × week; RT: 2 × week | CRP ↓, TNF-α ↓ |
| [54] | Australia | n – 47 M – 39 F – 8 | 48.1 | AE, RT, AE+RT | 12 weeks, 3 × week | CRP ↓, IL-6 ↓, TNF-α ↓ |
| [44] | USA | n – 22 M – 11 F – 11 | 42 | AE | 3 × week, 60 min at 60–85% HRR, 8 weeks | IL-6 ↓, IL-8 ↓, TNF-α ↓ |
| [37] | Saudi Arabia | n – 80 M – 40 F – 40 | 43.8 | AE | 12 weeks, 3 × week, 30–45 min at 60–70% HRmax | TNF-α ↓, CRP ↓, IL-6 ↓, IL-8 ↓ |
| [42] | China | n – 39 M – 20 F – 19 | 59.8 | AE | 10 weeks, 5 × week | IL-6 ↓ |
| [38] | Saudi Arabia | F – 80 | 52.6 | AE | 12 weeks, 3 × week, 30–45 min at 70% HRmax | TNF-α ↓, CRP ↓, IL-6 ↓ |
| [56] | Australia | n – 59 M – 45 F – 14 | 49.6 | AE, SIT | AE: 3 × week, 60 min at 80–85% HRmax; SIT: sprints | TNF-α ↓ IL-6 ↓ CRP ↓, IL-10 ↓ |
| [57] | Iran | n – 33 M – 22 F – 11 | 39.6 | NRT, AEI | 12 weeks, 3 × week | IL-18 ↓ IL-6 ↓ IL-10 ↑, CRP ↓, TNF-α ↓ |
| [52] | Italy | M – 16 | 58.5 | AE+RT | 12 weeks, 3 × week | TNF-α ↓, IL-6 ↓, CRP ↓ |
| [41] | Spain | n – 46 M – 23 F – 23 | 53.5 | AE | 24 weeks, 3 × week | CRP ↓ |
| [45] | Iran | F – 20 | 50.3 | AE | 12 weeks, 3 × week | TNF-α ↓, CRP ↓ |
| [43] | Lithuania | n – 126 M – 50 F – 76 | 53.3 | AE | 8 weeks, 5 ×/week | CRP ↓ |
| [49] | Iran | M – 24 | 53.9 | RT | 12 weeks, 3 × week | CRP ↓, IL-6 ↓, TNF-α ↓ |
| [32] | USA | M – 28 | 42.5 | INT, CONT | 2 weeks | LDL ↓, HDL ↑, TG ↓, VO2peak ↑ |
| [66] | Brazil | M – 39 | 42.5 | HIIT+RT | 12 weeks | LDL ↓, GLU ↓, INS ↓ |
| [60] | Brazil | M – 38 | 42.5 | HILIT | 12 weeks | TG ↓, HDL ↑, VO2 ↑, SBP ↔ DBP ↔ |
[i] M – male, F – female, LI – low-intensity, MI – moderate-intensity, HI – high-intensity, RA – resistance aerobic, AE – aerobic exercise, BE – balance exercise, HILIT – high-intensity low-volume interval training, HIIT – high-intensity interval training, RT – resistance training, SIT – sprint interval training, NRT – nutritional resistance training, AEI – aerobic exercise intermittent, WC – waist circumference, SBP-systolic blood pressure, DBP – diastolic blood pressure, HDL – high-density lipoprotein, LDL – low-density lipoprotein, TG – triglycerides, GLU – glucose, INS – insulin, CRP – C-reactive protein, IL-6 – interleukin-6, IL-8 – interleukin-8, TNF-α – tumour necrosis factor-alpha, IL-10 – interleukin-10, VO2max – maximal oxygen uptake, VO2peak – peak oxygen uptake