Introduction
The theory of resource sharing in motor and cog- nitive control states that the Central Nervous System shares the same resources to perform cognitive and motor tasks, i.e., cognitive tasks involve motor-cognitive interactions [1, 2]. The present study seeks evidence of this theory through an analysis of correlations be- tween motor and cognitive control in people with neu- rological injuries. Motor control is studied from the dimensions of postural control and upper extremity (UE) function and activity, whereas working memory (WM) is a variable of executive functions.
Following Horak’s conceptual model [3, 4], static and dynamic balance tests and dynamic postural con- trol are used to assess global postural control. This model assumes that postural control is the ability to maintain equilibrium in a gravitational field, and to stabilise the body’s centre of mass over its base of sup- port. The main functional objectives of this model are postural orientation and equilibrium. The former in- volves orientation of the torso and the head relative to the gravity vector, support surfaces, visual surround- ings, and internal references. Meanwhile, concerning the latter, postural equilibrium involves the coordina- tion of movement strategies to stabilise the body’s centre of mass in response to self-initiated and externally triggered disturbances of stability [4–6].
The approach to UE function and activity is consist- ent with the proposed assessment and foundations for clinical decision making by Lang et al. [7]. Function is the ability to perform activities with the upper limbs. In our study, motor activity is assumed from the pro- posal of Human Movement as a Complex System of the Universidad Autónoma de Manizales, Colombia. That is, as the ‘integration of multiple motor actions in a given context and depending on a task situation, charac- terized by the motor skill in its execution’ [8, 9]. Lang et al. focus their evaluation on two key factors: 1) Iden- tification of impairments that limit the normal range of motion, and 2) The level of activity limitations and participation restrictions resulting from these impair- ments, which are important in addressing complex issues such as quality of life. Among the main impair- ments are paresis, loss of dissociated movements, ab- normal muscle tone, and somatosensory disorders.
Executive functions, including WM, are directly associated with the UE function within the context of a motor activity [10]. Liang et al. consider executive functions as a set of cognitive skills that are involved in the control processes used in planning, organising, and carrying out complex goal-directed behaviours [11]. They have classified them into core functions (inhib- itory control, working memory and cognitive flexibil- ity) and higher-level functions (reasoning, planning, and problem-solving, amongst others).
WM is an executive function responsible for the collection of cognitive processes. These temporarily re- tain information in an accessible state suitable for car- rying out any mental task aimed at the performance of a routine goal-directed activity [12, 13]. Such processes generally consist of a transient storage and concurrent manipulation of information [10, 13].
Most of the studies that support the shared resource theory between cognitive and motor control use the dual-task paradigm in their design [1, 2, 14–17]. It has been shown that when cognitive tasks are performed and the person is exposed to a motor demand, the per- formance of the former decreases; and conversely, when motor tasks are performed and an additional cognitive demand is exposed, motor performance decreases. We have not found studies that correlate cognitive and motor control variables, particularly using cognitive electroencephalographic recordings, neurobehavioral assessment, and a comprehensive approach to assess- ing motor control that includes postural control.
The objective of the present study was therefore to search for evidence of the shared resource theory, through an analysis of correlations between motor control and WM in people with neurological injuries. We hypothesise that the better the performance in the neuropsychological and electrophysiological tests of WM (Working Memory Index of the Wechsler Adult Intelligence Scale IV, Trail Making Test – part B, Block- Tapping Test, and Cognitive Event-Related Potentials), the better the performance in motor control variables (balance, gait stability, grip strength, hand dexterity, daily life tasks, instrumental activities of daily living, and ability to grasp, transport and release). The study was conducted in people with neurological injuries to ensure that participants had varying levels of impaired motor control and to thus achieve the necessary vari- ability for a correlational analysis; a variability that is not wide enough in healthy people without alterations in motor control.
Materials and methods
Design
Following an analytical empirical approach, a de- scriptive and correlational scope study was conducted. Neuropsychological and electrophysiological variables of WM were correlated with two dimensions of motor control: postural control, and UE function and activity. Field work was conducted between August and Decem- ber 2021 at the Neurophysiology Laboratory of the Uni- versidad Autónoma de Manizales, Colombia.
The study was approved by the University Ethics Committee, recorded in the minutes 114 on the 28th of April 2021. This study is part of a macroproject on WM and motor control in adults with neurological injuries, which aims to provide empirical evidence on embodied cognition. All procedures were in accordance with the tenets of the latest Declaration of Helsinki and the guidelines of the Colombian resolution 8430 of 1993 of the Ministry of Health on human experimentation.
Sample and sampling
A non-probabilistic sampling of people with neuro- logical injuries was carried out. The sample size was calculated with the estimation formula for a linear association with a two-sided test, a confidence level of 0.95, a statistical power of 0.80 and an expected cor- relation of 0.37 [18], for a minimum sample size of 55 people. Subjects were recruited from rehabilitation cen- tres, sports teams for people with disabilities, founda- tions for people with disabilities, and students with neurological injuries at the Autonomous University of Manizales.
All participants met the following inclusion criteria: both sexes, age between 18 and 55 years old, neuro- logical injuries of any origin and body region, stable clinical condition, not hospitalised, with more than three months of having suffered the injury, affiliated to the Colombian health security system, and signature of a consent form. People over 55 years of age were not included to avoid the bias of possible cognitive decline typical of ageing.
Participants with any of the following conditions were excluded: Mini-Mental State Examination (MMSE) lower than 25 points out of 30; mental health condi- tions such as schizophrenia, anxiety, and bipolar dis- order [19, 20], alcoholism, substance abuse or any other type of addiction [21]. Lastly, hearing impairment greater than 30 dB measured by a clinical test and the presence of uncompensated visual impairment. The lack of motor control of the UE was not considered an exclusion criterion since these alterations are precisely one of the study variables. We sought to correlate dif- ferent degrees of alteration of UE motor control with possible variations in WM. In brain lesions, tests of UE function and activity were performed with the non- paretic UE.
Participants
Fifty-six individuals with neurological injuries be- tween the ages of 19 and 55 years with an average age of 38 years (SD = 11 years) participated. Most of them were men, single, with higher education, of low and middle socioeconomic status, and without employment or early retirement due to a disability. All participants reported having social security for healthcare cover- age, most of them in the contributory regime. Most were right-hand dominant and normal range of weight ac- cording to their body mass index (see Table 1).
Table 1
Characteristics of the participants (n = 56)
Forty-one people with neurological injuries of non- cerebral origin and 15 with a brain origin were eval- uated. The age of first occurrence, related to neuro- logical injuries, ranged from birth to 53 years, with a mean of 20 years and with an average progression of 17.8 years (see Table 2). Table 1 depicts the most rele- vant diagnoses associated with spinal cord injury, brain injury and neuropathy and peripheral nerve injury. People with myopathies, ataxias, focal dystonia, mul- tiple sclerosis, and amyotrophic lateral sclerosis also participated.
Instruments
Sociodemographic, anthropometric, and clini- cal data: survey and clinical history
Upper extremity function and activity [7, 22]
– Grip strength: digital dynamometer (Camry 90 kg professional digital hand dynamometer, model EH101)
– Ability to grasp, transport and release: Box and Block Test (BBT)
– Hand dexterity: Nine-Hole Peg Test (NHPT)
– Daily life tasks: Jebsen-Taylor Hand Function Test (JTT)
– Instrumental Activities of Daily Living (IADL):
Lawton and Brody Scale
Postural Control
Working memory
– Neurosychological Assessment: Working Mem- ory Index of the Wechsler Adult Intelligence Scale IV (WMI-WAIS IV), Trail Making Test – part B (TMT-B), and Corsi Block-Tapping Test (Corsi BBT)
– Neurophysiological evaluation using Cognitive Event-related Potentials (ERPs): P300 wave latency, and N200-P300 peak-to-peak amplitude [26, 27]
Procedure
All participants signed the consent form after being informed of the objective and the tests that would be conducted. Tests were administered during the morn- ing and always followed the same order: IADL, ERPs, Grip strength, Tinetti Balance, TUG, BBT, HNPT, JJT, TMT-B, Corsi BTT and WMI-WAIS IV, in accordance with the biosafety protocols.
The ERPs were registered in a Cadwell Sierra Wave 11.0® neurophysiograph following the 10/20 system. The protocol consisted of: (a) Cleaning of the area with an abrasive gel in the midline of the frontal, central and parietal regions in both mastoid processes; (b) Affixing electrodes with conductive paste (isolated ground in the frontal region, reference in the mastoid and active in the Fz, Cz and Pz channels); (c) Verification of imped- ance below 5 KΩ; otherwise, the areas needed to be cleaned again; (d) EEG activity is registered between 0.5 Hz and 50Hz; (e) Auditory odd-ball methodology with bilateral hearing aids, taking into account the following parameters: frequent stimulus 80%, audio type, tone mode, frequency 100 Hz, intensity 65 dB, and infrequent stimulus 20%, audio type, tone mode, frequency 3000 Hz, intensity 65 dB; (f) Visual odd- ball methodology with the following parameters: dis- tance of one metre between the monitor and the partici- pant; frequent stimulus 80% (presentation of reversible pattern without target stimulus) and infrequent stim- ulus 20% (small rhombus in the centre of the screen) for a total of 200 stimuli. A discrimination task was employed using a reversible pattern in a monochromatic checkerboard of 4 × 4; and g) Participants mentally counted the number of nonfrequent stimuli that they identified during each of the tests.
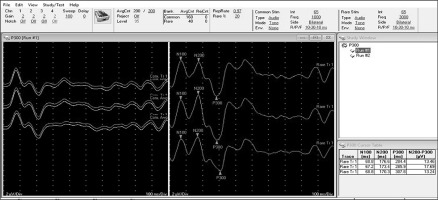
The Cadwell Sierra Wave 11.0® neurophysiograph, used in this study to measure ERPs, is fully parame- terised. It automatically averages the electroencephalo- graphic traces produced by the 200 stimuli, both audi- tory and visual, for each of the Fz, Cz, and Pz channels. The traces produced by frequent stimuli (80%) are shown on the left side of the screen, while the infrequent ones (20%) are observed on the right side (Figure 1). In the present research, the markings of the peaks of waves N100, N200 and P300 were made by one of the re- searchers and later corroborated by the other researcher. The latencies of the three waves and the N200-P300 peak-to-peak amplitude are automatically calculated by the equipment and displayed on the screen (Figure 1).
Figure 1
Illustration of Cognitive Event-related Potentials (ERPs), measureed with a Cadwell Sierra Wave 11.0® Neurophysiograph. On the left are the average electroencephalographic tracings produced by frequent stimuli (80%). On the right are the traces produced by infrequent stimuli (20%), in this case auditory. The lower right box shows the measurements taken automatically by the equipment for each of the channels (Fz, Cz and Fz in their order): wave latencies N100, N200 and P300, and peak-to-peak amplitude N200-P300
Statistical analysis
The statistical program SPSS version 27.0 (Statis- tical Package for the Social Sciences) was used for data processing. In all cases, two-tailed hypothesis tests were performed at a confidence level of 0.95 (p ≤ 0.05). No data was lost when gathering or analysing the data. The analysis of the UE motor control was performed using the DUE, even when it presented various levels of motor impairment. In cases of hemiparesis, the DUE was the opposite limb. Only one patient was unable to perform the JTT tests, and in their case, their values were imputed with the upper limit of the distribution.
The statistical analysis included: (a) Participant char- acteristics: univariate analysis of sociodemographic, clinical, and motor variables alongside WM (frequen- cies, median, range, mean, and standard deviation); (b) Performance on Kolmogorov–Smirnov tests, in which most variables do not exceed the assumption of nor- mality (p < 0.05), therefore, the correspondence tests were applied with nonparametric statistics; (c) Calcu- lation of Spearman’s correlation coefficient to assess postural control vs. WM; DUE function and activity vs. WM neuropsychological tests; and DUE function and activity vs. WM electrophysiological tests.
Results
Descriptive statistics of motor control and working memory tests
Table 2 shows the main descriptive statistics of pos- tural control, function, and activity of the dominant upper limb and WM tests (neuropsychological and electrophysiological tests).
Table 2
Descriptive tests of motor control and working memory (neuropsychological and electrophysiological tests) (n = 56)
[i] kp – kilopond (kilogram-force), NHPT – Nine Hole Peg Test, BBT – Block And Box Test, JTT – Jebsen-Taylor Hand Function Test, IADL – instrumental activities of daily living, NPT – neuropsychological tests, TMT-B, Trail Making Test, Corsi BTT – Corsi Block-Tapping Test, WAIS IV – Wechsler Adult Intelligence Scale, version IV, ERPs – event-related potentials, Fz, Cz and Pz – midline electrodes, frontal (F), central (C) and parietal (P)
Relationship between postural control and working memory
In general, the neuropsychological and the electro- physiological tests did not exhibit any statistically sig- nificant association between postural control and WM (p > 0.05). The significant associations (p < 0.05) dis- played between the Tinetti Balance Assessment Scale with the WAIS IV digit retention test and with the N200-P300 amplitudes with auditory stimulus in the Fz channel, as well as the TUG Test with the P300 wave latency with auditory stimulus in the Pz channel, can be considered mere coincidence (see Table 3).
Table 3
Correlations between postural control and working memory (neuropsychological and electrophysiological tests) (n = 56)
Relationship between Dominant Upper Limb Motor Control and neuropsychological working memory tests
TMT-B, BBT and JTT (p ≤ 0.05) displayed statis- tically significant low and moderate associations (see Table 4). These findings demonstrate that the better the performance (quickest time) in the TMT-B, the better the performance in the execution of the JTT (quickest time; rho = 0.258 to 0.466) and BBT (greater number of cubes transported; rho = –0.353) tests. The TMT-B (rho = 0.466) showed the strongest association with the ‘writing a short sentence’ test.
Table 4
Correlations between neuropsychological tests of working memory and dominant upper limb motor control (n = 56)
[i] rho – Spearman’s correlation coefficient, kp – kilopond (kilogram-force), TMT-B – Trail Making Test – Part B, Corsi BTT – Corsi Block-Tapping Test, WAIS IV – Wechsler Adult Intelligence Scale, version IV, WMI – Working Memory Index, NHPT – Nine Hole Peg Test, BBT – Box and Block Test, JTT – Jebsen-Taylor Hand Function Test, A – writing a short sentence, B – turning over a 3 × 5-inch card, C – picking up small common objects, D – simulated feeding, E – stacking checkers, F – picking up large light cans, G – picking up large heavy cans, IADL – Instrumental Activities of Daily Living Significant correlations are highlighted in bold (p < 0.05).
The backward span sequence of the Corsi BTT ex- hibited significant associations with four subtests and the overall average of the JTT (p ≤ 0.05) (see Table 4). The results demonstrate that the better the performance on the Corsi BBT (more correct sequences of tapped cubes), the better the performance (quicker time) on the subtests when writing a short sentence, the turn- ing over of a 3 × 5-inch card, the simulated feeding and picking up large light cans test, and the overall average of the JJT (rho = –0.260 to –0.323). The ‘simulated feeding’ test showed the strongest association with the Corsi BTT backward sequence (rho = –0.323).
The WAIS-IV showed a significant association with the JTT (p ≤ 0.050) (see Table 4). The findings show that the better the performance on the WAIS-IV (higher scores), the better the performance (quicker the time) on the JJT. There was a statistical correlation between the arithmetic test, and all the subtests and the overall average of the JTT (rho = –0.258 to –0.338). However, no correlation was found in the ‘stacking checkers’ test (p = 0.168). The total score of the WAIS-IV-digit retention test showed an association between simulat- ed feeding and the overall average score (rho = –0.281 and –0.274). Finally, The WMI had a significant as- sociation with the simulated feeding, the picking up large light cans, and the overall average score (rho = –0.273 to –0.321).
Grip strength, NHPT and IADL (p > 0.05) did not exhibit any significant associations with the neuro- psychological test of the WM.
Relationships between dominant upper limb motor control and electrophysiological tests of working memory
There were no statistically significant associations between the dominant upper limb function and activity with the P300 wave latency and N200-P300 amplitudes of Cognitive ERPs (p > 0.05) (see Table 5). However, the grip strength displayed an association with the N200- P300 amplitudes (auditory Pz and visual Cz channels). In summary, the main correlations found between WM and motor control in people with neurological injuries occurred with the variables of DUE function and activity. In general, no significant correlations were found between WM and global postural control.
Table 5
Correlations between electrophysiological tests of working memory and dominant upper limb motor control (n = 56)
[i] DUE – Dominant Upper Extremity, NHPT – Nine Hole Peg Test, BBT – Box and Block Test, JTT – Jebsen-Taylor hand function test, A – writing a short sentence, B – turning over a 3 × 5-inch card, C – picking up small common objects, D – simulated feeding, E – stacking checkers, F – picking up large light cans, G – picking up large heavy cans, IADL – Instrumental Activities of Daily Living, Fz, Cz and Pz – midline electrodes: frontal (F), central (C) and parietal (P) * p < 0.05
It was observed that the better the performance in WM in the neuropsychological tests, the better the per- formance in the DUE motor control tests, particularly in the Manual Activities of Daily Living (MADL). Sig- nificant correlations were also found between grip strength and the amplitude of the N200-P300 waves (electrophysiological test of WM): the greater the grip strength, the greater the N200-P300 peak-to-peak am- plitude that was observed.
Discussion
The purpose of the present study was to seek em- pirical evidence of the shared resource theory, through the study of correlations between motor control and WM in people with neurological injuries. It was hypoth- esised that the better the performance in the neuropsy- chological and electrophysiological tests of WM, the better the performance in the motor control variables.
In general, significant correlations were evident between DUE function and activity with the assessed neuropsychological tests of WM, in line with the work- ing hypothesis. However, no significant correlations were found between global postural control and WM, nor between DUE motor control with the latency of the P300 wave and the N200-P300 amplitude of the ERPs, except for grip strength. The above findings par- tially support the working hypothesis, with respect to the neuropsychological tests, but not with respect to the electrophysiological tests.
Working memory tests
From these results, it is striking that the standard deviation of the TMT-B is almost as large as the medi- an, with a coefficient of variation (CV) of 74%, a disper- sion explained by the heterogeneity of the population. This large dispersion is consistent with data reported in the Colombian population of the same age group, which have reported CVs of 57% and 40% [28, 29]. The mean of the present study is in the 50th percentile of the normative data of the healthy Colombian popu- lation [28].
Postural control and working memory
In the present study, no significant correlations were found between WM and global postural control. How- ever, it has been reported that, in the long term, execu- tive functions are significantly associated with tandem, semi-tandem, and single-legged stance [30]. These studies concluded that improving balance may reduce the risk of executive functions deteriorating. This evi- dence reflects how the common neural processes are shared between cognitive and motor areas of the cen- tral nervous system.
Achieving the full potential of postural control de- pends on the integration of visual, vestibular, proprio- ceptive, and tactile inputs. These are all important in the regulation of tonicity, force perception and pres- sure when maintaining balance [31]. These integration processes occur mainly based on automatic or semiau- tomatic mechanisms, guided by feedback and feed- forward strategies of the neuromuscular system [32], in which WM participates in the background [6, 33], except in cognitive-motor dual task activities in which it participates in an essential way [11, 34].
Regarding cognitive-motor dual-task training, it has been shown that these programs significantly improve walking balance, gait quality, and UE function [35]. However, in the present study, no cognitive tasks were performed during the global postural control test, which could explain the previously mentioned results.
Manual activities of daily living and working memory
The TMT-B, the most illustrative neuropsychologi- cal test in this correlate, showed significant associations with the overall average scores and with all MADL sub- tests of the JTT, except the stacking checkers test. The backward component of the Corsi BBT exhibited an association with the overall average scores and the sub- tests of the JJT of DUE: writing a short sentence, turn- ing over a 3 × 5-inch card, simulated feeding, and pick- ing up large light cans. Finally, the WAIS-IV exhibited an association with the overall average scores and sev- eral JJT subtests of DUE, especially in the arithmetic component, followed by the WMI.
In summary, associations were observed between behaviourally assessed WM and MADL. The findings demonstrate that the better the performance in WM, the shorter the time to perform the subtests and the general average of the JTT.
Consistent with these findings, other researchers have reported significant associations between MADL and cognitive functions assessed with neuropsycho- logical tests in different populations, such as motor competence and executive functions in typically de- veloping children [36], UE performance and executive functions in people with childhood-acquired stroke [37], manipulative task versus cognitive-motor plan- ning in children, adolescents, and young adults with cerebral palsy [38], audiovisual integration tasks, fin- ger dexterity, and bimanual coordination versus ex- ecutive functions in older adults with mild cognitive impairment [39].
Similarly, cognitive exercise therapy and cognitive- motor dual task training, based on activities of daily living, have been reported to significantly improve UE function, cognitive function, and quality of life in pa- tients with neurological injuries, compared with single- task training [40, 41].
These results invite researchers to explore func- tional recovery approaches in neurological disorders based on a combination of cognitive, perceptual, and motor strategies [41]. The variables affected by motor planning deficits should be considered to optimise functional rehabilitation, beyond merely biomechani- cal aspects [38]. In this way, the intimate interrelation- ship between UE performance and executive functions stands out, such that training complex and cognitively attractive motor skills that involve UE performance, rather than basic motor skills such as hand strength, during a rehabilitation program, may have the potential to foster the development of executive function and vice versa [37].
Improvement in UE function may be due to high-in- volvement activities such as sensory stimulation and movement of the upper extremities using the involved segments in daily life. Said improvement exerts a posi- tive effect on the speed of cognitive processing and con- sequently on the quality of life of the participants [40].
In this research framework, the concept of ‘instru- mental cognitive activities of daily living’ (ICADLs) ap-pears, which combines cognitive processes with the function and activity of people in the context of the mo- tor activities of people in a community. ICADLs are especially related to executive functions in everyday situations, such as managing household finances, at- tending social events, making monthly payments, shop- ping, and using public transportation. As a result, the ICADLs assess executive functions with ecological validity to predict individuals’ functioning [42].
In summary, UE function plays a crucial role in the performance of complex tasks of activities of daily living. These tasks involve WM and other executive functions and are closely related when participating in social activities [41]. From this perspective, the brain/ body-in-the-world system is displayed, which is the cognitive sciences’ object of study within the current dynamic paradigm [43].
Grip strength and working memory
In the present study, no WM neuropsychological test was significantly correlated with grip strength, but sig- nificant correlations were found between grip strength and the amplitude of the N200-P300 waves, observ- ing that the greater the grip strength, the greater the peak amplitude at peak N200-P300.
In contrast to our findings, other studies have re- ported significant associations between grip strength and cognitive functions, assessed with neuropsycho- logical tests, such as grip strength versus incident de- mentia, cognitive decline, and various cognitive func- tions in older people [44, 45]; grip strength and memory in people with post-intensive care syndrome [46]; and grip strength versus executive functions and speed of cognitive processing in people with stroke [40].
However, it is necessary to highlight that in these previous studies, there may be an interaction of con- founding variables such as ageing [44, 45], post-con- valescent cognitive-motor functional impairment [46], and the presence of concomitant cognitive and motor alterations in a health condition [40]. That is, the cor- relation found can be explained by a third variable that produces effects per se on WM memory and grip strength, thus creating a spurious correlation. These variables are not confusing in the present study since we worked with people between 18 and 55 years of age without cognitive decline or impairment. In addi- tion, most of the participants did not have a brain le- sion. It should be borne in mind that, in our study, all the participants exceeded 25 points in the MMSE.
Moreover, in contrast to neuropsychological tests, in the present study, grip strength was associated with electrophysiological tests (cognitive ERPs). Greater grip strength was associated with higher N200-P300 am- plitudes, indicative of better executive cognitive func- tioning [26, 27].
Consequently, previous studies have reported the same significant correlations in different populations with and without neurological injuries, such as in adults with myotonic dystrophy without genetically proven dementia [47], in children and adolescents with autism spectrum disorder [48], in athletes and healthy non- athletes [26], obese adults [49], and adolescents with diabetes mellitus [50], among others.
The underlying mechanisms remain unclear. How- ever, physical activity and fitness have generally been associated with favourable alteration of the P300 am- plitude of cognitive ERPs elicited by executive function tasks in healthy individuals [48]. Shang et al. concluded that grip strength training can improve cognitive func- tion by increasing the local efficiency of brain white matter connectivity. They suggest that white matter re- modelling is a potential physiological mechanism con- necting grip training and cognition enhancement [51]. These results encourage further exploration of grip strength as a predictor or indicator of WM impairment in people with and without neurological injuries.
Limitations
The present results do not provide conclusive em- pirical evidence of the cognitive-motor interaction, based on the study of relationships between WM and motor control of the DUE. However, there are strik- ing correlations between WM and DUE function and activity, especially referring to instrumental activities of daily living. The evidence is not conclusive since, contrary to our working hypothesis and in support of the null hypothesis, no significant correlations were found between WM and global postural control (static and dynamic balance), nor between DUE motor con- trol with electrophysiological measures (latency of the P300 wave and the N200-P300 amplitude of the ERPs), except for grip strength. On the other hand, consistent with our working hypothesis, significant correlations were evident between the function and activity of the DUE (ability to grasp, transport and release, and daily life tasks) and the assessed neuropsychological tests of WM (WAIS IV, TMT-B and Corsi BBT). These find- ings require further investigation.
Additionally, the present study is limited by the sample size and the variety of health conditions of the participants.
Conclusions
Future research with larger samples and with more defined populations, as well as with populations with motor impairment without neurological compromise, may yield more conclusive results on the correlations explored here. Despite the above, and preliminarily, the significant relationships between WM and DUE function and activity found here, especially referring to instrumental activities of daily living, encourage the conjugation of cognitive and motor interventions in the rehabilitation of people with neurological and cog- nitive impairments.


