Introduction
Epidemiological studies show that stroke is the most common disease of the nervous system and is the second most common cause of mortality worldwide and the third most common cause of neuromotor disability [1]. After a stroke, more than 70% of patients remain long-term mobility disabled, and 30% of patients, due to severe mobility impairment, remain under the constant care of others. Given that the incidence of stroke increases with age, and life expectancy in societies is lengthening, it is not difficult to see the magnitude of the problem we will soon face. the rapid rate of increase in the percentage of the elderly in the human population should be a signal to entire societies and government institutions to pay attention to the health problems of this increasingly numerous, and most helpless and doomed social group. Stroke patients exhibit poor outcomes that include re-hospitalisation (33%), recurrent event (7–13%), dementia (7–23%), mild cognitive disorder (35–47%), depression (30–50%) and fatigue (35–92%). In Europe, the incidence of stroke ranges from 95 to 290 per 100,000 per year, with a 13 to 35% fatality rate per month, which is also rising amongst young adults [2].
There is an urgent need for resources that mini-mise the public health burden, and to include preventive strategies that efficiently reduce stroke in young individuals [3]. to meet the challenges facing society, various forms and methods are being sought to improve the functioning of both the elderly and post-stroke people. on the other hand, there is a need to clarify the molecular mechanisms of change underlying the therapeutic process, for a better understanding of the problem of plasticity in post-stroke patients and the introduction of new and improved methods based on empirical grounds.
Stroke is a traumatic neurological event caused by a vascular-circulatory derangement that results in parenchymal lesions. Whether by a rupture in blood vessels with intracerebral or intra-ventricular haemorrhage due to a lack of or insufficient blood oxygen supply [4–6], neural damage is caused by severe local oxidative stress, disruption of the plasma membrane and organelle swelling and leakage of cellular contents into the extracellular space. these events initiate intrinsic and extrinsic pathways for cell death and necrosis in the surrounding neural tissue [7, 8].
From another perspective, the endogenous regulation of brain-derived neurotrophic factor (BDNF), a neurotrophic factor with a major role in nervous system protection, will exert a relevant impact on the cost of stroke to the populational quality of life. While cardiometabolic diseases that silently develop become the leading cause of stroke worldwide, regulation of BDNF, which is metabolically responsive, provides resources to a more prompt recovery from eventual damage to neural tissue [9].
BDNF is a small protein expressed from the gene BDNF as a pre-proBDNF mrNA transcript that is translated into an active proBDNF protein isoform then cleaved into the mature BDNF protein [10]. BDNF auto-crine and paracrine actions via binding to tyrosine kinase (trkB) receptors at the cell membrane surface are essential to synaptic function and the formation of neural circuits, and they guarantee neuronal plasticity and pro-survival mechanisms [11]. the precursor proBDNF form, instead, interacts with p75 receptors and regulates neuronal apoptosis – the precise control of BDNF/proBDNF release dictates BDNF’s actions in the development and functioning of the nervous system [12–14]. In addition, a single-nucleotide change (or polymorphism) from G to A at nucleotide position 196 in the protein coding sequence of the BDNF gene and subsequent change in amino acid from valine to methionine at position 66 (e.g., Val66Met) rs6265 in the pro-domain of the proBDNF [15, 16] occurs in about 10–12% of the general population, with higher rates being associated with poor outcomes in the subpopulation with neuropsychiatric conditions [17–20].
It is important to understand that rehabilitation from a stroke usually requires a long period and strong dedication, and does not always lead to full recovery; and that this is even more unfortunate for young individuals due to the risk of living with stroke outcomes for the majority of their lives. Inclusion of aerobic exercise in the scope of stroke-oriented rehabilitation programs might benefit the time cost and quality of recovery. We have shown that high-intensity interval training (HIIt) ameliorates BDNF levels as it improves cardiorespiratory fitness [21]. Now, such strategies seem to have begun to be explored in stroke patients, with good prospects. Here, in this literature review, we have summarised the clinical evidence of the influence of BDNF on the recovery from a stroke and discuss the potential inclusion of exercise in rehabilitation programs.
Material and methods
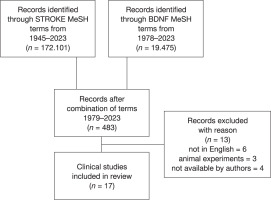
In order to retrieve all the available data on BDNF analyses in stroke patients, a literature search was conducted in PubMed databases using multiple combinations of the Medical Subject Headings (MeSH) terms for ‘StroKE’ and ‘BDNF’ (Figure 1). Stroke referring to a group of pathological conditions characterised by sudden, non-convulsive loss of neurological function due to brain ischemia or intracranial haemorrhages, and the search terms included: ‘brain infarction’, ‘cerebral infarction’, ‘hemorrhagic stroke’, ‘ischemic stroke’, ‘embolic stroke’ and ‘thrombotic stroke’; and BDNF also included the ‘pro-BDNF’ pro-neurotrophin supplementary concept. Four hundred and eighty-three papers resulted from combinations of the search terms updated on June 30th of 2023. After the search was filtered for clinical trials and duplicates were removed, thirty papers were retrieved and screened for inclusion in the discussion according to criteria. of those, six studies were excluded for not being available in the English language, three studies referred to animal experiments, and four of the five studies requested from their authors were not made available before manuscript submission. the included studies should present data on serum BDNF or BDNF gene polymorphism in patients affected by stroke events. Seventeen clinical studies, including a short report, were included for discussion in this review. this study is part of the project “the impact of training to improve patients’ condition after ischemic stroke” registered at the International Standard randomised controlled trial Number, No ISrct N16891871.
Role of BDNF in stroke
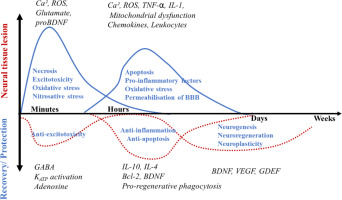
Neural recovery from stroke is a complex phenomenon that includes intense neuroinflammation. the damage caused to neural tissue involves glutamate leakage, intracellular accumulation of calcium and apoptosis. Upon lesion, there is a local increase in extra-cellular glutamate excitotoxicity and oxidative-nitrosative stress, which leads to neuroinflammation and permeabilisation of the blood-brain barrier, which may last from a few hours to days. the study by Mollet et al. [22] summarises the molecular events in the pro-inflammatory and anti-inflammatory phases of a stroke (see Figure 2).
In parallel, pro-inflammatory cytokines such as interleukin (IL) 1 and tNF- rapidly increase to evoke phagocytic activity and the expression of anti-inflammatory factors such as the anti-apoptotic factor B-cell lymphoma 2 (Bcl-2) and BDNF. BDNF signalling through trkB in glial cells induces proliferation and differentiation of astrocytes and neuroblasts that migrate to the lesion region to contribute to tissue repair and help form glial scar tissue. the recovery role of neuroblasts also involves the secretion of BDNF to the microenvironment of the damaged tissue [23, 24], wherein neuronal activation of trkB by BDNF promotes survival via the ca2+ balance against glutamate excitotoxicity, reducing inflammation and apoptosis and increasing Bcl-2 expression [25–28]. the anti-inflammatory role of BDNF thus prevails in the final phase of recovery.
Results
Influence of BDNF Val66Met (rs6265) polymorphism
The BDNF gene presents a single nucleotide polymorphism – a guanosine to adenosine variation – that results in a valine (Val) swift to methionine (Met) in the pro-region of the translated proBDNF protein, named ‘Val66Met’, which has a higher incidence in sub-populations with neuropathologic conditions [29, 30]. In our previous research, we showed that the BDNF Val66Met polymorphism might negatively influence BDNF secretion by affecting different stages of its synthesis. It affects cleavage and trafficking processing of proBDNF throughout the secretory pathways, resulting in a less efficient distribution of BDNF in the dendrites and axons or its activity-dependent release from synaptic terminals [31, 32].
The influence of BDNF on stroke outcomes was first reported in patients of haemorrhagic stroke (31 Met carriers and 65 Val homozygous) investigated by Vilkki et al. [33] when they noticed that Met carriers showed inferior learning and memory capability one year after stroke (table 1). chronic stroke patients (42 Val homozygotes and 30 Met carriers) carrying the Met-allele exhibited a deficit in motor skills compared to the Val homozygotes during the recovery phase [34]. Likewise, stroke patients with unilateral motor weakness (9 Val homozygotes and 37 Met carriers) in a study by chang et al. [35] exhibited greater motor improvement from combined therapy with transcranial stimulation when they did not carry the polymorphism.
Table 1
Data summary of the clinical studies available in PubMed databases
When Fridriksson et al. [36] included transcranial stimulation in the recovery therapy of chronic stroke patients (37 Val homozygotes and 30 Met carriers), it was noticed that the Val homozygotes patients’ recovery was improved only when transcranial stimulation was combined to their therapy; whereas the Met carriers showed improvement from both traditional and combined therapy. Such differential responsiveness might occur due to a subtle negative influence of the BDNF Val66Met polymorphism on BDNF activity that benefits more from external stimuli. When Essa et al. [37] evaluated the effect of electrical stimulation in stroke patients with dysphagia (7 Val homozygotes, 9 Val/Met heterozygotes and 1 Met homozygote), the Met carriers showed greater benefits in dysphagia at three months but not at two weeks of treatment.
In a study by Mirowska-Guzel et al. [38], transcranial stimulation did not have a different effect on serum BDNF levels or aphasia recovery between controls and ischemic stroke patients with aphasia (11 Met carriers and 35 Val homozygotes), and the patients with post-stroke dysmnesia (14 Val homozygotes vs 11 Met homozygotes vs 15 Val/Met heterozygotes) from a study by Lu et al. [39] saw no effect of transcranial stimulation on BDNF and cognitive/motor functions regardless of genotype. While the levels of BDNF correspond to the capability of recovering from a stroke, differences between genotypes are not so obvious across the patient population and do not determinate prognosis.
The investigation performed by Pascotini et al. [40] addressed the relationship between the Ala16Val polymorphism in the manganese-dependent superoxide dismutase (MnSoD) gene, which encodes for a major antioxidant enzyme defence within mitochondria, and serum BDNF levels in stroke patients, and found that MnSoD Val homozygotes have lower BDNF levels and higher pro-inflammatory markers, and are more frequent amongst stroke patients.
BDNF levels as a neuroprotection cue
Blood levels of BDNF are found decreased in different neurodegenerative conditions [41–43]. A study by Algin et al. [44] reports that there is a difference in blood levels of BDNF between non-stroke and stroke patients. their study showed that the level of BDNF in patients with acute ischemic stroke is 3.89 ± 2.05 ng/mL, and this is significantly lower than those of age-matched healthy subjects: 14.9 ± 4.7 ng/mL.
The ability of neurons to synthesise BDNF, in times referred to as the ‘cognitive reserve’, is crucial during neural tissue recovery from a traumatic event or when coping with neurodegenerative disease. Patients with post-stroke cognitive impairment assigned to rehabilitation combined with transcranial stimulation in a study by Wang et al. [45] showed that the blood BDNF levels and memory performance increased with both treatments, and were higher in the combined treatment. Data from a study by Asadollahi et al. [46] also presented that a higher serum BDNF level accompanies better neurological recovery in stroke patients. the cellular synthesis of BDNF increases with the oxidative metabolism and so it is more efficient when cells display of higher mitochondrial response to oxidative stress. As mitochondria have their own DNA, they continually adapt in shape and quantity to cover for higher energetic challenges and antioxidant defence [9, 47]. Along this line of thinking, there seems to be a role for BDNF in preventing strokes, observing a negative association between serum BDNF with the risk of stroke/transient ischemic attack in individuals from a 10-year community-based cohort study [48].
A study by El-tamawy et al. [49] showed that the inclusion of aerobic exercise in an 8-week physiotherapy program induced an increase in serum BDNF levels and cognitive capacities in ischemic stroke patients. Later on, a study by Morais et al. [50] showed that a single session of 30 minutes of walking at a moderate intensity of 64–76% of maximum heart rate increases the serum BDNF levels in chronic post-stroke patients.
In a study by King et al. [51], chronic stroke patients were submitted to an incremental test for maximum aerobic exercise capacity on a body-weight supported treadmill, and the results showed that their intelligence score was associated to their physical activity and changes in BDNF levels after testing. thereafter, when Ploughman et al. [52] paired cognitive interventions (computerised dual working memory training [coG] or control computer games [Games]) to two low-intensity exercise routines: Aerobic (> 60% Vo2peak using < 10% body weight-supported treadmill) or Ac-tivity (range of movement and functional tasks), the results showed that intelligence scores improved in the Aerobic + coG group but not in the Activity + coG group in relation to Activity + Games; however, there was no effect of the interventions on BDNF.
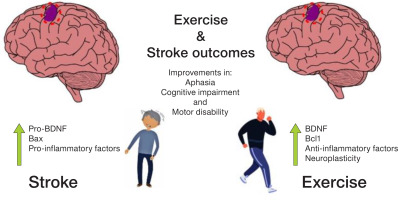
Exploring potential mechanisms involved in cognitive impairment presented by patients with stroke, Wang et al. [53] tested incrementing various cognitive training strategies (attention, thinking, memory and orientation) in stroke patients during rehabilitation. Patients with increments to their rehabilitation showed improvements in cognitive-motor parameters gathered with serum BNDF and the anti-apoptotic factor Bcl-2, and lower pro-inflammatory and pro-apoptotic factors (Figure 3). Specifically investigating depression in stroke patients, a pilot study by Palumbo et al. [54] measured the serum BDNF and oxytocin levels in patients with post-stroke hemiparesis in either a group music-making intervention program or an individual recovery program. Data showed that participants who exhibited reduced depression had increased levels of serum BDNF. Likewise, in the study by cui and Wei [55], acupuncture combined with traditional treatment for post-stroke depression resulted in lower depression scores and higher serum BDNF levels.
A trial by Boyne et al. [56] evaluated the effect of different exercise protocols, which included a treadmill and seated-stepper at different intensities, on the serum BDNF levels in stroke patients with unilateral paresis. the results showed that exercising at high intensity induces greater increases in BDNF levels, and that blood lactate accumulation is important in eliciting a BDNF response. A study by Hsu et al. [57] that compared the effect of moderate-intensity continuous and HIIt on serum BDNF levels in stroke patients confirmed that HIIt induces a greater increase in cardiorespiratory fitness (Vo2max) and serum BDNF levels than moderate-intensity exercise in these patients.
Should exercise be included in rehabilitation from stroke?
There are numerous benefits of exercise for the brain’s health. In particular, the contribution of BDNF as an exercise-inducible neuroprotective factor with the capability to repair and maintain neuronal function is well-described in the literature. However, recognising exercise as a matter of public health is still a major challenge, especially in developing countries [58–60]. only two studies have addressed the influence of exercise on BDNF in the recovery from a stroke; both of which confirmed that moderate- to high-intensity exercise evokes adaptive changes in the oxidative metabolism that include neuronal readiness to synthesise BDNF and neuroplasticity improvement during stroke recovery [61–64].
Similarly to what is described in neurodegenerative conditions [65–67], the changes in BDNF levels found throughout the studies’ data generally represented the patients’ capability to recover from the cognitive and motor control deficits that result from stroke events. Furthermore, the BDNF Val66Met polymorphism appears to influence patients’ neuroplasticity. Moreover, while differences in BDNF levels between Val homozygotes and Met carriers are not detectable, the incidence of individuals carrying the Met-allele amongst patients with stroke appears higher than those seen in healthy individuals. the expression of BDNF is mainly regulated by synaptic activity so that cell-type and tissue-specific secretion patterns might selectively be affected by the BDNF Val66Met polymorphism in a temporal and spatial manner. therefore, although differences in serum BDNF levels among individuals with different BDNF genotypes are not noticeable, a vulnerability to disturbances in the nervous system in those who carry the Met-allele appears consistent with scientific literature reports [15, 16, 68–71].
From a neuroprotective perspective, there is a positive effect of exercise on BDNF actions in patients with stroke, like those seen in healthy individuals, reported as improvements in the patients’ recovery of cognitive and neuromotor functions.
Nevertheless, the inclusion of exercise as a clinical prescription in stroke rehabilitation does not seem to be a short-term reality, as the power of exercise is still underestimated in therapeutic strategies. In this regard, future research addressing such patients should consider the proposal of sufficiently standardised protocols with a detailed description of patients’ features and limitations and exercise methods, in order to provide a solid background for the potential use of exercise in clinical rehabilitation. Apart from the type, volume and intensity, which should also be discussed in future studies, exercise can be an economic resource for stroke patients’ rehabilitation and such an impact on the public health budget should be addressed. In this sense, the exercise-induced maintenance of BDNF levels might potentially have an influence on stroke outcomes [31, 72].
Highlights and final considerations
It came to our attention that, whereas the common Val66Met polymorphism in BDNF gene is reported to affect several processes of the protein synthesis [32], this is not directly reflected in the circulating serum levels [29, 31]. this leads to the interpretation that a slight misfunctioning in such an essential protein for the proper neuronal function is tolerable, although it does represent a vulnerability to the development of neuropathological conditions. In the case of traumatic events such as stroke, the studies’ data are quite consistent in reporting a negative relation between neuro-regenerative power and the presence of the Met-allele in BDNF, as grouped by homozygotes and heterozygotes.
Exercise, on the other hand, is a potential therapeutic strategy in the recovery of stroke outcomes via modulation of the pro-neuroplasticity and neuronal survival actions of BDNF. It can shorten the rehabilitation period in a manner that Met-carrier individuals should benefit the most and improve traditional rehabilitation and therapeutic approaches. Nevertheless, the inclusion of exercise as a clinical recommendation still requires a deeper understanding of the physiological and molecular mechanisms to which patients might be exposed in different exercise types and protocols, and considering individual limitations related to the condition.